Mead acid (20 carbon atoms), named after James F. Mead, the research at UCLA who first identified it in 1959, belongs to the group of unsaturated fatty acids, having three cis double bounds (the first one from the methyl end is in omega-9 (ω-9) or n-9, so in shorthand in named 20:3n-9). It is also a member of the sub-group called very long chain fatty acids (VLCFA), from 20 carbon atoms onwards.
PROPERTIES
Molecular weight: 306.48276 g/mol
Molecular formula: C20H34O2
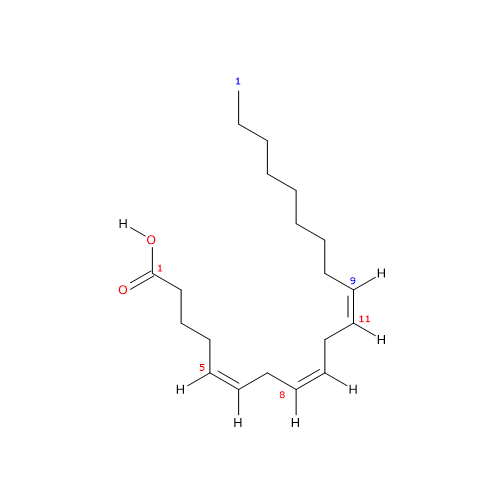
IUPAC name: (5Z,8Z,11Z)-icosa-5,8,11-trienoic acid
CAS registry number: 20590-32-3
PubChem: 5312531
OTHER NAMES
5Z,8Z,11Z-eicosatrienoic acid
cis-5,8,11-eicosatrienoic acid
20:3n-9
Synthesis of Mead acid
In animals, it can be produced de novo from oleic acid (18:2n-6).
The enzymes that catalyze the conversion of oleic acid (18:1n-9), linoleic acid (18:2n-6) and alpha-linolenic acid (18:2n-3) to the C20 n-9, omega-6 polyunsaturated fatty acids and omega-3 polyunsaturated fatty acids are the same but omega-6 and omega-3 families have greater affinity for them than does the omega-9; from a biochemical point of view this is competitive inhibition. In essential fatty acid deficiency competitive inhibition is lack and enzymes will desaturate oleic acid to 18:2n-9 (Δ6 desaturase), which is further elongated and Δ5 desaturated to form Mead acid.
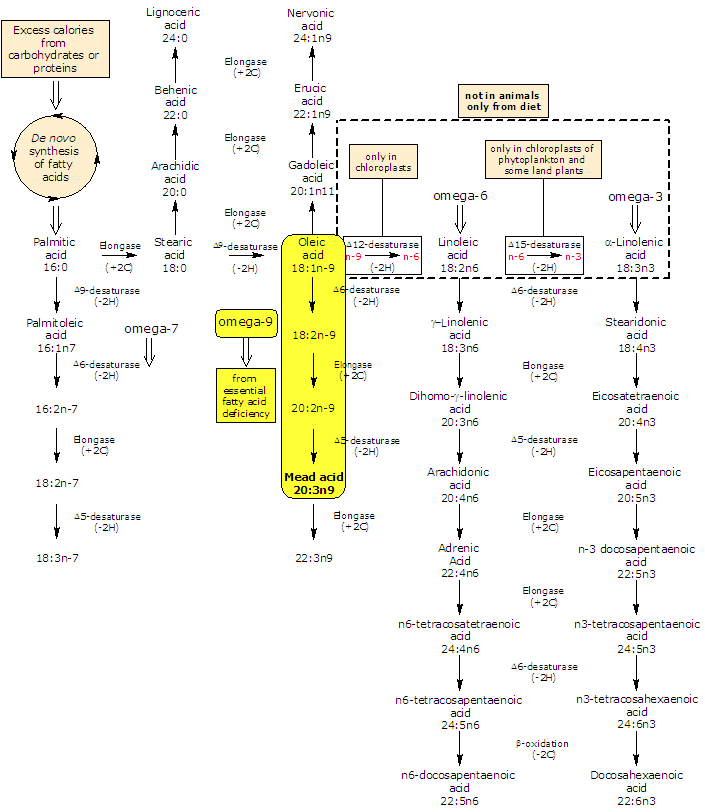
Essential fatty acid deficiency
Its elevated presence in the blood is an indication of essential fatty acid (EFA) deficiency.
It has been speculated that in essential fatty acid deficiency the desaturation of oleic acid is a cell attempt to replace arachidonic acid in membrane with similarly unsaturated species. Although it would be incorporated into the same tissues and complex lipids as arachidonic acid, it cannot replace it or alleviate the symptoms of essential fatty acid deficiency. Moreover, it is not a substrate for the CycloOXygenase (COX) reaction, and no eicosanoids are produced from it.
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008