The Cori cycle was discovered by Carl Ferdinand Cori and Gerty Theresa Radnitz, a husband-and-wife team, in the ‘30s and ‘40s of the last century. They demonstrated the existence of a metabolic cooperation between the skeletal muscle working under hypoxic conditions and the liver.[1]
The cycle allows the conversion of lactate, the conjugated base of lactic acid and its dominant form at physiological pH, into glucose, thus ensuring a continuous supply of the monosaccharide to peripheral tissues. The importance of this cycle is also demonstrated by the fact that it is responsible for about 40 percent of plasma glucose turnover.[7][8]
From a biochemical point of view, the Cori cycle links gluconeogenesis with anaerobic glycolysis, using different tissues to compartmentalize opposing metabolic pathways.[10]
This metabolic cooperation was demonstrated to exist also between other extrahepatic tissues and liver. Indeed, like the glucose-alanine cycle, the Cori cycle is active between the liver and all those tissues that do not completely oxidize glucose to CO2 and H2O.[11]
Contents
Steps
The analysis of the steps of the Cori cycle is made considering the lactate produced by red blood cells and muscle fibers.[12]
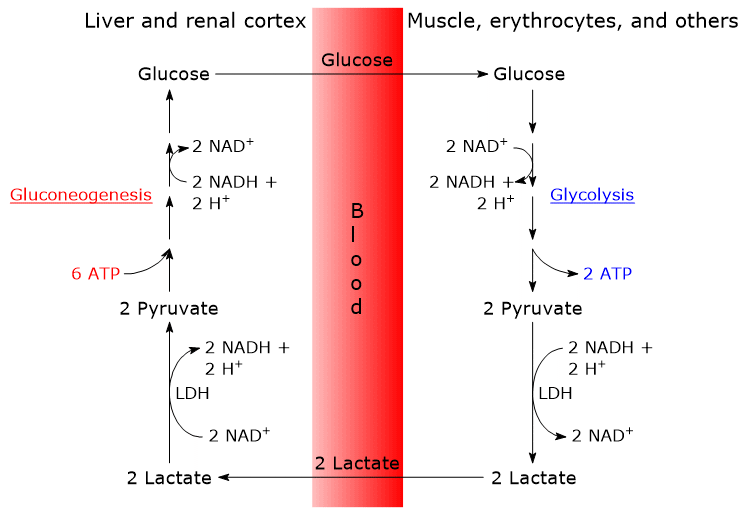
- The cycle begins with the conversion of glucose to lactate, through anaerobic glycolysis and the subsequent action of lactate dehydrogenase (EC 1.1.1.27), which catalyzes the reduction of pyruvate, the conjugate base of pyruvic acid.
- This is followed by the diffusion of lactate from the cell into the bloodstream, by which it is transported to the liver, its main user, and the renal cortex, in particular the proximal tubules, as these are another site where gluconeogenesis occurs.
- In the liver and renal cortex, lactate is oxidized to pyruvate, in a reaction catalyzed by lactate dehydrogenase. Pyruvate is then converted to glucose by the gluconeogenic pathway.[8]
- Finally, glucose diffuses into the bloodstream and reaches red blood cells or muscle fibers, closing the cycle.[10][11]
Red blood cells
Mature red blood cells, being devoid of a nucleus, ribosomes, and mitochondria, are smaller than most other cells. The small size allows them to pass through tiny capillaries, but the lack of mitochondria makes them completely dependent on anaerobic glycolysis for ATP production. This means that these cells continuously produce lactic acid.[8][10]
The availability of NAD+ is essential for glycolysis to proceed as well as for its rate. Indeed, the oxidized form of the coenzyme is required for the oxidation of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate in the reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12).
Glyceraldehyde 3-phosphate + NAD+ → 1,3-Bisphosphoglycerate + NADH + H+
The accumulation of NADH is avoided by the reduction of pyruvate to lactate, in a reaction catalyzed by lactate dehydrogenase, where NADH acts as reducing agent, oxidizing to NAD+.[4]
Muscle fibers
Fast-twitch muscle fibers contain a reduced number of mitochondria and, under hypoxic conditions, such as during intense exercise, produce significant amounts of lactic acid.[8] In fact, in such conditions:
- the rate of pyruvate production by glycolysis exceeds the rate of its oxidation by the citric acid cycle, so that less than 10 percent of the pyruvate enters the citric acid cycle;
- the rate at which oxygen is taken up by the cells is not sufficient to allow aerobic oxidation of all the NADH produced.[3]
In such conditions, anaerobic glycolysis leads to the production of 2 ATP per molecule of glucose, 3 if the glucose comes from muscle glycogen, therefore, much lower than the 29-30 ATP produced by the complete oxidation of the monosaccharide.[9] However, the rate of ATP production by anaerobic glycolysis is greater than that produced by the complete oxidation of glucose.[10]
Finally, as in red blood cells, the reaction catalyzed by lactate dehydrogenase, regenerating NAD+, allows glycolysis to proceed, but produces lactate.[4]
Lactate
Lactic acid is an end product of metabolism that must be converted back into pyruvate to be used.[3]
The plasma membrane of most cells is freely permeable to both pyruvate and lactate, that can thus reach the bloodstream.[3] And, regarding for example the skeletal muscle, the amount of lactate that leaves the cell is greater than that of pyruvate due to the high NADH/NAD+ ratio in the cytosol and to the catalytic properties of LDH isozyme present in the muscle fibers.[5]
Once into the bloodstream, lactate reaches, among others, the liver and the renal cortex, where it is oxidized to pyruvate, in the reaction catalyzed by tissue-specific isozymes of lactate dehydrogenase.
In the hepatocyte, this oxidation is favored by the low NADH/NAD+ ratio in the cytosol. Then, pyruvate can enter gluconeogenesis, the next step of the Cori cycle, to be converted to glucose.[3]
Glucose enters into the bloodstream and is delivered to the muscle and red blood cells, thus closing the cycle. Obviously the monosaccharide also reaches all other tissues and cells that require it.
Energy cost
The Cori cycle results in a net consumption of 4 ATP.
The gluconeogenic leg of the cycle consumes 2 GTP and 4 ATP per molecule of glucose synthesized, namely, 6 ATP.
ATP-consuming reactions are catalyzed by:
- pyruvate carboxylase (EC 6.4.1.1): one ATP;
- phosphoenolpyruvate carboxykinase (EC 4.1.1.32): one GTP;
- glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12): one ATP.
Since two molecules of lactate are required for the synthesis of one molecule of glucose, the net cost is 2 x 3 = 6 high energy bonds per molecule of glucose.[2]
Conversely, the glycolytic leg of the cycle produces only 2 ATP per molecule of glucose.
Therefore, more energy is required to produce glucose from lactate than that obtained by anaerobic glycolysis in extrahepatic tissues. This explains why the Cori cycle cannot be sustained indefinitely.
Is the Cori cycle a futile cycle?
The continuous breakdown and resynthesis of glucose feature of the Cori cycle might seem like a waste of energy. In reality, this cycle allows the effective functioning of many extrahepatic cells at the expense of the liver and the renal cortex. Therefore, it is more correct to define it as a substrate cycle rather than a futile cycle.[10] Here below, some examples.
The Cori cycle allows to dispose of part of the lactic acid that red blood cells produce.[8]
Under intense exercise conditions, anaerobic glycolysis represents a primary means of ATP production for muscle fibers. But this could lead to an intracellular accumulation of lactate, and a consequent reduction in intracellular pH. Obviously, such accumulation does not occur, also thanks to the Cori cycle, in which the gluconeogenic tissues pay the cost of the disposal of a large part of the muscle lactate.[3] And the oxygen debt which occurs after an intense exercise is largely due to the increased oxygen demand of the hepatocytes, in which the oxidation of fatty acids, their main fuel, provides the ATP required for gluconeogenesis.[6][12]
During trauma, sepsis, burns, or after major surgery, an intense cell proliferation occurs in the wound, that is a hypoxic tissue, and in bone marrow. This in turn results in higher production of lactic acid, an increase in the flux through the Cori cycle, and an increase in ATP consumption in the liver, which, as mentioned, is supported by an increase in the oxidation of fatty acids. A similar condition seems to occur also in cancer patients with progressive weight loss.[2]
The Cori cycle is also important during overnight fasting and starvation.[6]
Cori cycle and glucose-alanine cycle
There are similarities and differences between the two cycles.
The glucose-alanine cycle and the Cori cycle are metabolic pathways that, through the intermediacy of the bloodstream, extend across different organs and help ensure a continuous supply of glucose to tissues.[12] In both cycles entry into gluconeogenesis involves the conversion of lactate and alanine into pyruvate. Finally, in both cycle, the glucose produced is then transported to peripheral tissues where the glycolytic pathway regenerates pyruvate.[13]
Their main difference lies instead in the three-carbon intermediate that is recycled: in the Cori cycle the carbon is returned to the liver as pyruvate, whereas in the glucose-alanine cycle it is returned to the liver as alanine.[8] Additionally, the metabolic fate of NADH also differs: in the Cori cycle it acts as a reducing agent in the reaction catalyzed by lactate dehydrogenase, whereas in the glucose-alanine cycle the electrons of NADH are used for the synthesis of ATP in the mitochondrion. Therefore, another difference is that the glucose-alanine cycle requires the presence of oxygen, while the Cori cycle does not.[8]
References
- ^ American Chemical Society National Historic Chemical Landmarks. Carl and Gerty Cori and Carbohydrate Metabolism. https://www.acs.org/education/whatischemistry/landmarks/carbohydratemetabolism.html
- ^ a b Bender D.A. Introduction to nutrition and metabolism. 3rd Edition. Taylor & Francis, 2004
- ^ a b c d e Berg J.M., Tymoczko J.L., and Stryer L. Biochemistry. 5th Edition. W. H. Freeman and Company, 2002
- ^ a b Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010
- ^ Gleeson T.T. Post-exercise lactate metabolism: a comparative review of sites, pathways, and regulation. Annu Rev Physiol 1996;58:565-81. doi:10.1146/annurev.ph.58.030196.003025
- ^ a b Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012
- ^ National Center for Biotechnology Information. PubChem Pathway Summary for Pathway WP1946, Cori cycle, Source: WikiPathways. https://pubchem.ncbi.nlm.nih.gov/pathway/WikiPathways:WP1946. Accessed June 12, 2024
- ^ a b c d e f g Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- ^ Rich P.R. The molecular machinery of Keilin’s respiratory chain. Biochem Soc Trans 2003;31(Pt 6):1095-105. doi:10.1042/bst0311095
- ^ a b c d e Rosenthal M.D., Glew R.H. Medical biochemistry – Human metabolism in health and disease. John Wiley J. & Sons, Inc., Publication, 2009
- ^ a b Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 3rd Edition. Elsevier health sciences, 2012
- ^ a b c Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011
- ^ Wu G. Amino acids: biochemistry and nutrition. CRC Press, 2010