The phenomenon that two or more different chemical compounds have the same molecular formula is called isomerism, from the Greek isos meaning “equal”, and meros meaning “part”, a concept and term introduced by the Swedish scientist Jacob Berzelius in 1830.[3][4]
Isomerism is a consequence of the fact that the atoms of a molecular formula can be arranged in different ways to give compounds, called isomers, that differ in physical and chemical properties.
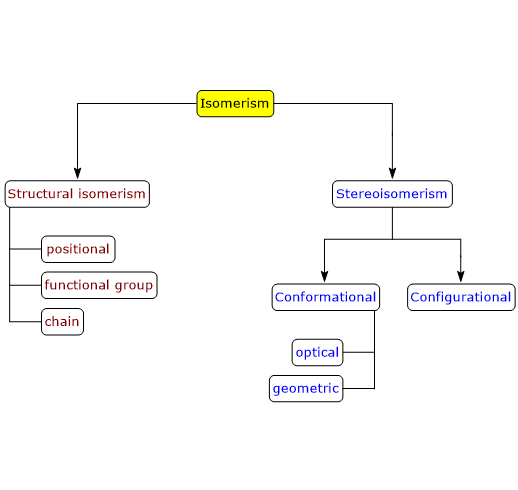
There are two types of isomerism: structural isomerism and stereoisomerism, which can be divided into further subtypes.[1]
Contents
Structural isomerism
In structural isomerism, also called constitutional isomerism, isomers differ from each other in that the constituent atoms are linked in different ways and sequences.
There are several subtypes of structural isomerism: positional, functional group and chain isomerism.
Positional isomers
In positional isomerism, also called position isomerism, isomers have the same functional groups but in different positions on the same carbon chain.
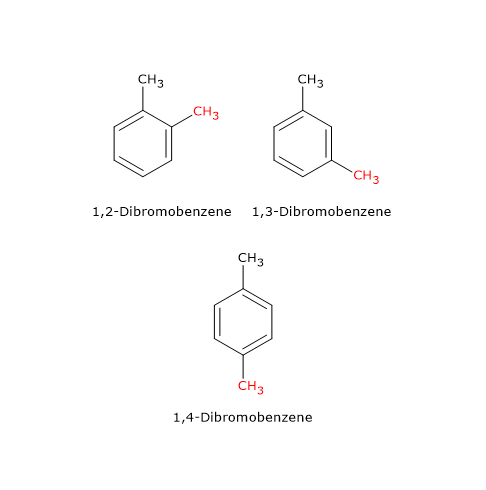
An example is the compound with molecular formula C6H4Br2, of which there are three isomers: 1,2-dibromobenzene, 1,3-dibromobenzene and 1,4-dibromobenzene. These isomers differ in the position of the bromine atoms on the cyclic structure.
Another example is the compound with molecular formula C3H8O, of which there are two isomers: 1-propanol or n-propyl alcohol, and 2-propanol or isopropyl alcohol. These isomers differ in the position of the hydroxyl group on the carbon chain.
Functional group isomers
Functional group isomerism, also called functional isomerism, occurs when the atoms form different functional groups.
An example the compound with molecular formula C2H6O, of which there are two isomers: dimethyl ether and ethanol or ethyl alcohol, that have different functional groups, an ether group, –O–, and a hydroxyl group, –OH, respectively.
Chain isomers
In chain isomerism, isomers differ in the arrangement of the carbon chains, that may be branched or straight.
An example is the compound with the molecular formula C5H12, of which there are three isomers: n-pentane, 2-methylbutane or isopentane and 2,2-dimethylpropane or neopentane.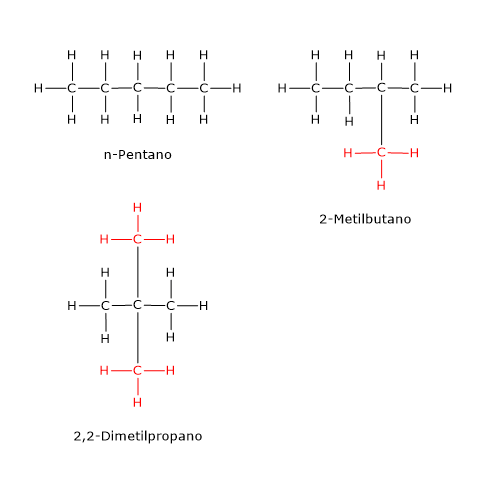
Chain isomerism also occurs in lipids. For example, among short-chain fatty acids, butyric acid and isobutyric acid, which have molecular formula C4H8O2, are chain isomers, as well as valeric acid, isovaleric acid and 2-methylbutyric acid, which have the molecular formula C5H10O2.
Stereoisomerism
In stereoisomerism, isomers have the same number and kind of atoms and bonds, but differ in the orientation of the atoms in space.[3][4] Such isomers are called stereoisomers, from the Greek stereos, meaning “solid”.
There are two subtypes of stereoisomerism, conformational isomerism and configurational isomerism; the latter can be further subdivided into optical isomerism and geometrical isomerism.
Conformational isomerism
In conformational isomerism, the stereoisomers can be interconverted by rotation around one or more single bonds, the σ bonds. These rotations produce different arrangements of atoms in space that are non-superimposable. And the number of possible conformations a molecule can adopted is theoretically unlimited, ranging from the lowest energy structure, the most stable, to the highest energy structure, the less stable. Such isomers are called conformer.
For example, if we consider ethane, C2H4, looking at the molecule from one end down the carbon-carbon bond, using the Newman projection, hydrogen atoms of a methyl group can be, with respect to the hydrogen atoms of the other methyl group, in one of the following conformations.
- The eclipsed conformation, in which hydrogen atoms of a methyl group are hidden behind those of the other methyl group, then, the angle between carbon-hydrogen bonds on the front and rear carbons, called a dihedral angle, could be 0, 120, 240, 360 degrees. This is the highest energy conformation, then is the less stable.
- The staggered conformation, in which hydrogen atoms of a methyl group are completely offset from those of the other methyl group, namely, dihedral angles could be 60, 180 or 360 degrees. This is the lowest energy conformation, then the most stable.
- The skew conformation, corresponding to one of the intermediate conformations between the previous ones.
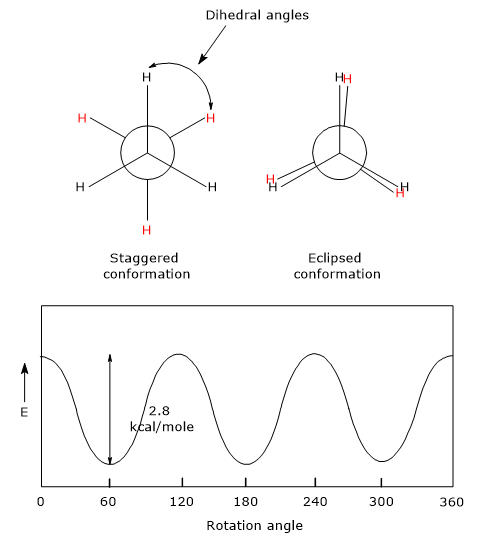
The stability of ethane conformers is due to how the electron pairs of the carbon-hydrogen bonds of the two methyl groups are overlapped:
- in the staggered conformations they are as far away from each other as possible;
- in the eclipsed conformations they are as close as possible to each other.
The potential energy barrier between these two conformations is small, about 2.8 kcal/mole (11.7 kJ/mole). At room temperature, the kinetic energy of the molecules is 15-20 kcal/mole (62.7-83.6 kJ/mole), more than enough to allow free rotation around the carbon-carbon bond. As a consequence, it is not possible to isolate any particular conformation of ethane.
Note: the potential energy barrier to rotation around double carbon-carbon bonds is about 63 kcal/mole (264 kJ/mole), corresponding to the energy required to break the π bond. (See geometric isomerism). This value is about three times the kinetic energy of the molecules at room temperature at which, then, free rotation is precluded. Only at temperatures above 300 °C molecules acquire enough thermal energy to break the π bond, allowing free rotation around the remaining σ bond. This allows the trans-isomer to be rearranged to the cis-isomer or vice versa.
Configurational isomerism
In configurational isomerism, the interconversion between the stereoisomers does not occur as a result of rotations around single bonds but involves bond breaking and new bond forming, then it doesn’t occur spontaneously at room temperature.
There are two subtypes of configurational isomerism: optical isomerism and geometrical isomerism.
Optical isomers
Optical isomerism occurs in molecules that have one or more chirality centers or chiral centers, namely, tetrahedral atoms that bear four different ligands.[2] The chiral center can be a carbon, phosphorus, sulfur or nitrogen atom.
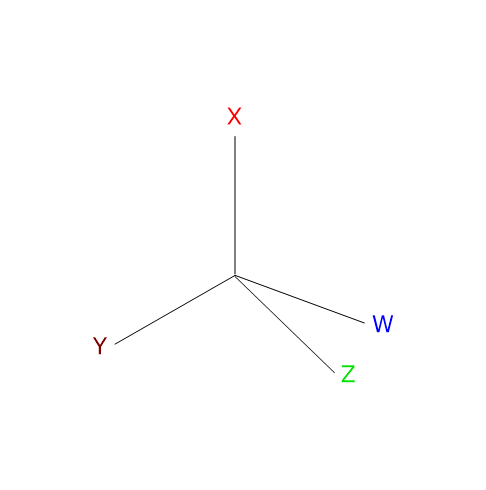 Note: the word chirality derives from the Greek cheiros, meaning “hand”.
Note: the word chirality derives from the Greek cheiros, meaning “hand”.
Optical isomers lack of a center of symmetry or a plane of symmetry, are mirror image of each other, and cannot be superimposed on one another. Such stereoisomers are called enantiomers, from the Greek enántios, meaning “opposite”, and meros, meaning “part”.
Unlike the other isomers, two enantiomers have identical physical and chemical properties with two exceptions.
- The direction of rotation of the plane of polarized light, hence the name of optical isomerism.
If a solution of one enantiomer rotates the plane of polarized light in a clockwise direction, the enantiomer is labeled (+). Conversely, a solution of the other enantiomer rotates the plane of polarized light in a counterclockwise direction by the same angle, and the enantiomer is labeled (-). - Although indistinguishable by most techniques, two enantiomers can be distinguished in a chiral environment like the active site of chiral enzymes.
Note: for a molecule with n chiral centers, the maximum number of stereoisomers is equal to 2n.
Geometric isomers
Geometric isomerism, also called cis-trans isomerism, occurs when atoms cannot freely rotate due to a rigid structure such as in:
- compounds with carbon-carbon, carbon-nitrogen or nitrogen-nitrogen double bonds, where the rigidity is due to the double bond;
- cyclic compounds, where the rigidity is due to the ring structure.
An example of geometrical isomerism due to the presence of a carbon-carbon double bond is stilbene, C14H12, of which there are two isomers. In one isomer, called cis isomer, the same groups are on the same side of the double bond, whereas in the other, called trans isomer, the same groups are on opposite sides.
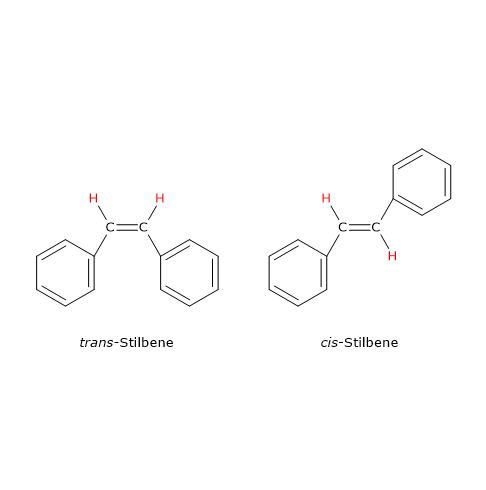
Note: the terms trans and cis are from the Latin trans, meaning “across”, and cis, meaning “on this side of”.
Among the cyclic compounds of carbon, cis-trans isomerism not complicated by the presence of chiral centers occurs in structures with an even number of carbon atoms and substituted in opposite positions, namely, para-substituted. An example is 1,4-dimethylcyclohexane, a cycloalkane, compounds of general formula CnH2n, of which there are two stereoisomers, cis-1,4-dimethylcyclohexane and trans-1,4- dimethylcyclohexane.
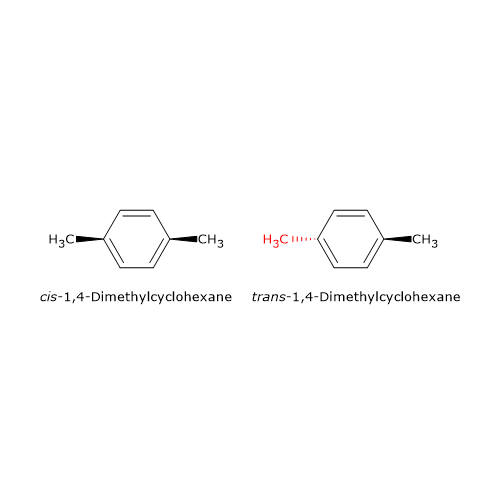
This kind of stereoisomerism cannot exist if one of the atoms that cannot freely rotate carries two groups the same. Why? For the switching between the trans and cis isomers the groups attached to atoms that cannot freely rotate have to be swapped. If there are two groups the same, the switch leads to the formation of the same molecule.
Note: geometric isomers are a special case of diastereomers or diastereoisomers, that, in turn, are stereoisomers that are not mirror image of each other. The other diastereomers are the meso compounds and non-enantiomeric optical isomers.
References
- ^ Graham Solomons T. W., Fryhle C.B., Snyder S.A. Solomons’ organic chemistry. 12th Edition. John Wiley & Sons Incorporated, 2017
- ^ Kelvin WT. Baltimore lectures on molecular dynamics and the wave theory of light. Clay C. J., London: 1904:619. https://archive.org/details/baltimorelecture00kelviala/mode/2
- ^ a b Morris D.G. Stereochemistry. Royal Society of Chemistry, 2001. doi:10.1039/9781847551948
- ^ a b North M. Principles and applications of stereochemistry. 1th Edition. CRC Press, 1998