In 1906, the Russian-American chemist Martin André Rosanoff, working at that time at New York University, chose glyceraldehyde, a monosaccharide, as the standards for denoting the stereochemistry of molecules with at least one chirality center, such as carbohydrates. This nomenclature system was called the Fischer-Rosanoff convention, or Rosanoff convention, or D-L system.[7]
Because Rosanoff didn’t know the absolute configuration of glyceraldehyde, he assigned in a completely arbitrary manner:
- the D prefix, from the Latin dexter, meaning “right”, to (+)-glyceraldehyde, the dextrorotatory enantiomer, thus assuming that the configuration, in Fischer projections, was that with the hydroxyl group (–OH) attached to the chiral center on the right side of the molecule;
- the L prefix, from the Latin laevus, meaning “left”, to (-)-glyceraldehyde, the levorotatory enantiomer, thus assuming that the configuration, in Fischer projections, was that with the hydroxyl group attached to the chiral center on the left side of the molecule.[4]
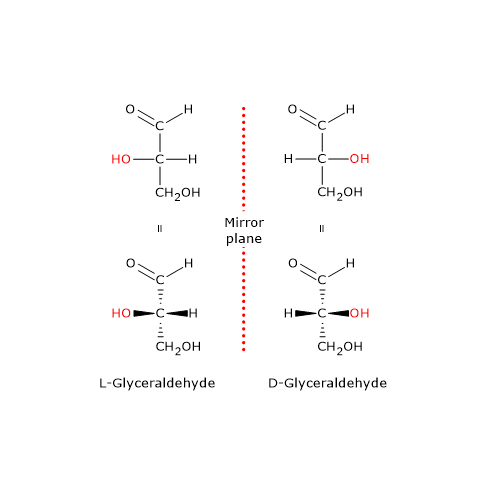
Although Fischer rejected this nomenclature system, it was universally accepted and used to obtain the relative configurations of the chiral molecules.[3] How? The configuration about a chiral center is related to that of glyceraldehyde by converting its groups to those of the monosaccharide through reactions that occur with retention of configuration, namely, reactions that do not break any of the bonds to the chiral center. This means that the spatial arrangement of the groups around the chiral center in the reagents and products is same. The Fischer convention allows to divide the chiral molecules, such as amino acids and monosaccharides, into two classes, known as the D series and the L series, depending on whether the configuration of the groups around the chiral center is related to that of D-glyceraldehyde or L-glyceraldehyde.
Note: there is no correlation between retention of configuration and sign of the rotatory power: the D-L system does not specify the sign of the rotation of plane-polarized light caused by the chiral molecule, but simply correlates the configuration of the molecule with that of the glyceraldehyde.[6]
Contents
- Fischer-Rosanoff convention and carbohydrates
- Fischer-Rosanoff convention and alpha-amino acids
- Relative and absolute configurations
- Ambiguities of the Fischer-Rosanoff convention
- References
Fischer-Rosanoff convention and carbohydrates
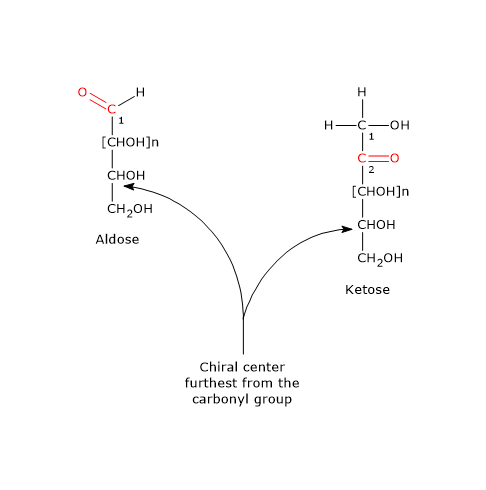
Monosaccharides can be aldoses or ketoses. Aldoses, and ketoses with more than three carbon atoms have at least one chiral center, and, by convention, they belong to the D series or to the L series if the configuration of the chiral carbon farthest from the carbonyl carbon, the carbon with the highest oxidation state, is same as that of D-glyceraldehyde or L-glyceraldehyde, respectively.
In Fischer projections the longest chain of carbon atoms is oriented vertically, and the atoms are numbered so that the carbonyl carbon has the lowest possible number, then, C-1 in aldoses and C-2 in ketoses.[8]
 Note: in Nature, D-sugars are much more abundant than L-sugars.
Note: in Nature, D-sugars are much more abundant than L-sugars.
If the sign of the rotation of plane-polarized light must be specified in the name, the prefixes (+) or (-) can be employed in addition to the D and L prefixes. For example, fructose, which is levorotatory, can be named D-(-)-fructose, whereas glucose, which is dextrorotatory, can be named D-(+)-glucose.
Fischer-Rosanoff convention and alpha-amino acids
Amino acids, depending on the position of the amino group (–NH2) with respect to the carboxyl group (–COOH) can be classified as:
- α-amino acids, in which the amino group is attached to the α-carbon;
- β-amino acids, in which the amino group is attached to the β-carbon;
- γ-amino acids, in which the amino group is attached to the γ-carbon;
- δ -amino acids, in which the amino group is attached to the δ-carbon.
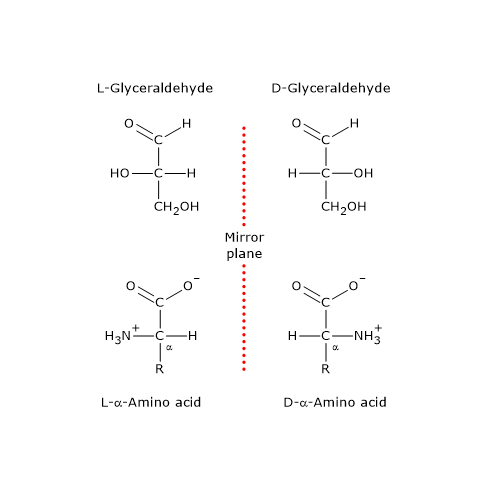
α-Amino acids belong to the D series or to the L series if the configuration of the –NH2, –COOH, –R, and H groups attached to the α-carbon, the chiral center, is the same of the hydroxyl, aldehyde (–CHO), and hydroxymethyl (–CH2OH), and H groups of D-glyceraldehyde or L-glyceraldehyde, respectively.[6][8]
In Fischer projections the molecules are arranged so that the carboxylic group, namely, the carbon with the highest oxidation state, is at the top, and the R group at the bottom.
Among α-amino acids, proteinogenic amino acids, with the exception of glycine whose α-carbon is not chiral, have the L configuration, hence, they are L-α-amino acids.
Note: in Nature, L-α-amino acids are much more abundant than all the other amino acids, which do not participate in the synthesis of proteins.
Relative and absolute configurations
When Rosanoff arbitrarily assigned the D prefix to (+)-glyceraldehyde and the L prefix to (-)-glyceraldehyde, he had 50/50 chance of being correct.[5]
In the early 1950s, a new technique, the x-ray diffraction analysis, made possible to establish the absolute configuration of chiral molecules. In 1951 a Dutch chemist, Johannes Martin Bijvoet established the absolute configuration of sodium rubidium (+)‐tartrate tetrahydrate and, comparing it with glyceraldehyde, demonstrated that Rosanoff’s guess was right.[1] Consequently, the configurations of the chiral compounds obtained by relating them to that of glyceraldehyde were the same as their absolute configurations: hence, the relative configurations became absolute configurations.
Ambiguities of the Fischer-Rosanoff convention
The Fischer-Rosanoff convention gives rise to uncertainties with molecules with more than one chiral center.[3] For example, considering D-(+)-glucose, the D-L system gives information about the configuration of C-2, but no information about the other asymmetric centers, namely, C-3, C-4, and C-5.
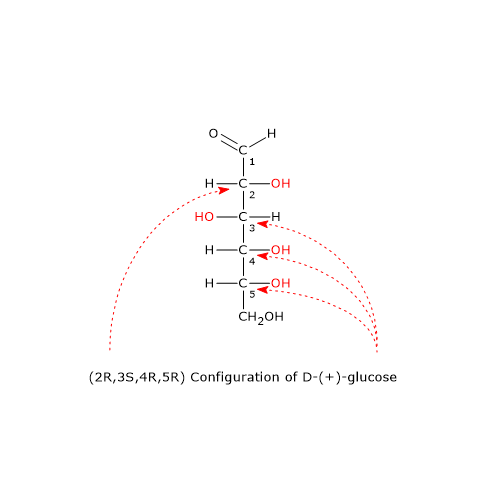
In these cases, the RS system, developed in 1956 by Robert Sidney Cahn, Christopher Ingold, and Vladimir Prelog, labeling each chiral center, allows to describe accurately the stereochemistry of the molecule.[2][8] In the case of D-(+)-glucose, the molecule has the (2R,3S,4R,5R)-configuration.
It should also be noted that depending on the chiral center taken as the reference center, the same molecule can belong to both the D and L series.
References
- ^ Bijvoet J.M., Peerdeman A.F., Van Bommel A.J. Determination of the absolute configuration of optically active compounds by means of X-rays. Nature 1951;168(4268):271. doi:10.1038/168271a0
- ^ Cahn R.S., Ingold C., Prelog V. Specification of molecular chirality. Angew Chem 1966:5(4); 385-415. doi:10.1002/anie.196603851
- ^ a b Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010
- ^ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. doi:10.1351/goldbook
- ^ Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012
- ^ a b Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- ^ Rosanoff. On Fischer’s classification of stereo-isomers. J Am Chem Soc 1906:28(1);114-121. doi:10.1021/ja01967a014
- ^ a b c Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011