Amylose is a polysaccharide composed of α-D-glucose units linked by α-(1→4) glycosidic bonds, with a few branches connected to the main chain by α-(1→6) glycosidic bonds.[1]
Together with amylopectin, it is one of the two main constituents of starch, the major storage form of energy and carbohydrates in the biosphere.[2][3]
Its synthesis is catalyzed by the enzyme granule-bound starch synthase (GBSS; EC 2.4.1.242) and requires the presence of a second protein, known as protein targeting to starch 1 (PTST1), which has no catalytic activity.[4]
Within the starch granule, amylose is embedded in the semi-crystalline matrix formed by amylopectin.[5] Unlike amylopectin, amylose is not essential for the formation of starch granules, is present in smaller amounts, but exerts a strong influence on the physicochemical properties of starch.[6][7]
Plant varieties have been developed whose starch granules contain either negligible amounts or, conversely, very high amounts of amylose. These phenotypes have both industrial applications and potential health benefits.[8][9][10]
Contents
- Structure
- Amylose location in starch granules
- Synthesis
- Amylose/amylopectin ratio
- High-amylose starches
- References
Structure
Amylose molecules have a molecular weight of about 106 daltons. They are mostly linear and composed of α-D-glucose units (hereinafter referred to as glucose) linked by α-(1→4) glycosidic bonds, that is, covalent bonds between the C-1 atom of one unit and the hydroxyl group on the C-4 atom of the next unit.[11] The linear chains are composed of several hundred to several thousand monosaccharides; therefore, they are much longer than amylopectin chains.[1]
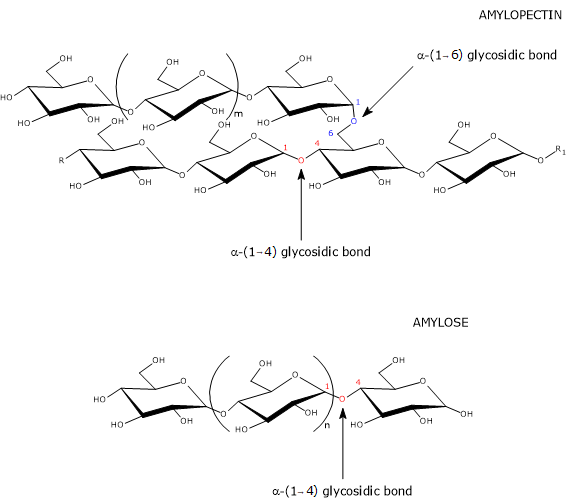
The few branches are connected to the linear chain by α-(1→6) glycosidic bonds, as in amylopectin and glycogen, the storage form of carbohydrates in animals. An α-(1→6) glycosidic bond is a covalent bond between the C-1 atom of one unit and the hydroxyl group on the C-6 atom of another glucose unit. The number of branches ranges from 5 to 20, depending on the botanical origin of the starch. Unlike those of amylopectin, the branches are not clustered.[12][13]
Studies on the length of amylose branches have shown a bimodal distribution, with two fractions termed:
- AM1, comprising shorter chains, with a degree of polymerization between 100 and 700;
- AM2, comprising longer chains, with a degree of polymerization between 700 and 40,000.[14]
A similar bimodal distribution of branch length is also observed in amylopectin, whose fractions are termed AP1 (shorter and more abundant) and AP2. The intraspecies variation in the distribution of AM1 and AM2 fractions is relatively small, whereas the variation among different species is considerable and has a genetic basis.[14]
Amylose location in starch granules
The precise location of amylose within starch granules is not fully known, although it is generally believed to be concentrated in the amorphous regions. However, some studies suggest that its distribution is not limited to these regions, but that amylose can also be found interspersed between amylopectin chains and on the surface of the granules. Thus, amylose may occupy multiple locations within the granule.[5][15]
Synthesis
In plants, the synthesis of amylose is catalyzed by GBSS, one of the six isoforms of starch synthase.[16] This enzyme, whose activity is the major determinant of amylose content in starch granules, requires the presence of the PTST1 protein.[4] Amylose branching appears to be carried out by the starch branching enzyme (SBE; EC 2.4.1.18).[17]
As in the case of amylopectin biosynthesis, it is believed that the enzymes involved physically interact to form multienzyme complexes, which optimize the efficiency of the process.[1] Because amylose synthesis requires a pre-existing amylopectin matrix to target GBSS to the starch granules, the synthesis of the two polysaccharides is not entirely simultaneous.[16]
Priming and initiation of amylose synthesis
In the initial steps, GBSS, like other starch synthases, requires short malto-oligosaccharides (MOS), α-(1→4) glucans with a degree of polymerization of 2 to 7, which act as primers for chain elongation.[17] MOS can originate from various sources, such as the trimming of nascent amylopectin molecules by starch debranching enzymes, or the activity of starch phosphorylase (EC 2.4.1.1), another enzyme involved in starch metabolism.[18] Because MOS are poorly soluble in water, they appear able to evade the hydrolytic activity of α-amylase (EC 3.2.1.1) and β-amylase (EC 3.2.1.2), and diffuse within the starch granule matrix, where they are elongated by GBSS.[19] Once malto-oligosaccharides are elongated beyond seven glucose residues, they can no longer diffuse out of the granule and are further extended in situ.[20]
Since a primer is required, the early stages of amylose and amylopectin biosynthesis resemble those of glycogen. However, in glycogen synthesis the primer is the self-glucosylating protein glycogenin.[21]
Importantly, the polymerization of glucose into amylopectin, amylose, glycogen, and, more generally, the conversion of osmotically active monosaccharides into osmotically inactive polysaccharides, enables the storage of large amounts of carbohydrate within the cell without increasing osmotic pressure.[21][22]
Granule-bound starch synthase
GBSS, together with the other isoforms of starch synthase and starch phosphorylase, belongs to the glycosyltransferase family (EC 2.4).[16]
GBSS was discovered by Louis Federico Leloir’s group, which had previously identified the main pathway for galactose, known as the Leloir pathway. It is the most abundant protein associated with starch granules.[23] Unlike the other isoforms of starch synthase, which are mostly located in the plastid stroma or partitioned between starch granules and the stroma, GBSS is found almost exclusively bound to granules. Protease treatment of the starch granule surface has shown that most GBSS is located within the granule rather than on its surface, a position consistent with amylose synthesis inside nascent granules.[4]
In grasses, GBSS is present in two isoforms encoded by distinct genes. These isoforms are:
- GBSSI, present in the amyloplasts of storage tissues (non-photosynthetic tissues);
- GBSSII, present in chloroplasts (photosynthetic tissues), where it participates in the synthesis of transient starch.[24]
GBSS catalyzes the transfer of a glucose residue from ADP-glucose to the non-reducing end of an α-(1→4)-glucan, forming a new α-(1→4) glycosidic bond.[21][25]
(1→4)-α-D-glucosyl(n) + ADP-α-D-glucose ⇌ (1→4)-α-D-glucosyl(n+1) + ADP + H+
Notably, starch synthases use ADP-glucose as the glucosyl donor, whereas glycogen synthase, the enzyme responsible for glycogen synthesis, uses UDP-glucose.[22][26]
Granule-bound starch synthase can add more than one glucose monomer per substrate encounter, a processive mode of action. This contrasts with the other isoforms of starch synthase, which add only a single glucose unit per encounter, a distributive mode of action. The processive activity of GBSS enables the biosynthesis of long linear chains and appears to be strongly enhanced by the presence of amylopectin.[1]
PTST1
Amylose synthesis requires the presence of a protein from the PTST family, namely PTST1, which was discovered more than fifty years after GBSS.
PTST1 has no catalytic activity but enables the binding of GBSS to the starch granule. This function appears to be more important in chloroplasts, and therefore in the synthesis of transient starch, than in amyloplasts.
It has been proposed that PTST1 associates with GBSS in the plastid stroma. The complex then binds to the nascent starch granule, after which PTST1 dissociates, allowing GBSS to initiate amylose synthesis. PTST1 subsequently returns to the plastid stroma to recruit another GBSS molecule. The importance of PTST1 is highlighted by its conservation throughout the plant kingdom. Loss of PTST1 causes GBSS to detach from the nascent starch granule, thereby preventing amylose synthesis.[4][19]
Starch branching enzymes
The enzyme responsible for generating the few α-(1→6) linkages present in amylose molecules is not definitively known, although an isoform of starch branching enzyme, SBEI, may be involved.[5]
SBEI is located primarily in the plastid stroma, with only a small proportion bound to starch granules, and is mainly expressed in storage tissues. The low frequency of branching in amylose may result from its biosynthesis inside the granules, where SBEI is scarce, thereby protecting the nascent molecule from the enzyme’s action.[27]
Amylose/amylopectin ratio
In the starch granules of land plants, amylose is almost always present in variable amounts, generally between 5% and 35%. Variability occurs not only among species but also within the same species, depending on the organ or tissue examined. In tubers and seeds, the amylose content also varies with the stage of development: it is generally low in the early stages and increases until the final value is reached, a pattern consistent with the synthesis of amylose within an amylopectin matrix.[5][25]
However, some plants have starch with very low, or even no, amylose content. This type of starch is referred to as waxy, owing to the raw endosperm’s appearance, which resembles wax. Conversely, certain plants produce starch granules composed mostly, or even entirely, of amylose.[9]
Although its precise role is not yet fully understood, the near-constant presence of amylose suggests that this polysaccharide plays an important structural role in starch granules and confers some advantage to plants during growth and development.[5]
The amylose/amylopectin ratio strongly influences the physicochemical properties of starch. For example, it affects water absorption, which in turn influences processes such as starch retrogradation and gelatinization, as well as resistance to enzymatic hydrolysis. This determines, for instance, the rate at which maltose and maltotriose are released during starch degradation by α-amylase or β-amylase.[28] These properties, in turn, influence both the industrial applications of starch and its effects on human health.[10]
High-amylose starches
High-amylose cereals can be obtained by enhancing GBSSI gene expression or by suppressing or eliminating the genes encoding starch branching enzymes, SSIIa, or other enzymes and proteins involved in amylopectin biosynthesis. However, in cereal endosperm, the most effective method is the suppression or elimination of one or more starch branching enzyme isoforms.[9]
High-amylose starches exhibit distinctive physicochemical properties, such as high gelling strength, easy retrogradation, and excellent film-forming ability. These properties make them suitable for industrial applications, including the production of biodegradable plastics, paper, and adhesives.[6][28]
High-amylose starches also contain elevated levels of resistant starch, a form of starch that escapes digestion in the small intestine by α-amylase, one of the key hydrolases involved in carbohydrate digestion. Studies with resistant starch–enriched foods have shown improvements in insulin sensitivity and glycemic response, as well as a reduced risk of cardiovascular disease, obesity, and type II diabetes mellitus.[29]
Mechanism of action
Resistant starch, by escaping intestinal digestion, lowers the glycemic index of foods in which it is present, thereby helping regulate blood glucose levels. Once it reaches the colon, it is fermented by the gut microbiota, which is part of the human microbiota, producing short-chain fatty acids (mainly butyric acid, acetic acid, and propionic acid), which play an essential role in maintaining intestinal health.[30][31]
References
- ^ a b c d Tetlow I.J., Bertoft E. A review of starch biosynthesis in relation to the building block-backbone model. Int J Mol Sci 2020;21(19):7011. doi:10.3390/ijms21197011
- ^ Qu J., Xu S., Zhang Z., Chen G., Zhong Y., Liu L., Zhang R., Xue J., Guo D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci Rep 2018;8(1):12736. doi:10.1038/s41598-018-30411-y
- ^ Apriyanto A., Compart J., Fettke J. A review of starch, a unique biopolymer – Structure, metabolism and in planta modifications. Plant Sci 2022;318:111223. doi:10.1016/j.plantsci.2022.111223
- ^ a b c d Seung D., Soyk S., Coiro M., Maier B.A., Eicke S., Zeeman S.C. PROTEIN TARGETING TO STARCH is required for localising GRANULE-BOUND STARCH SYNTHASE to starch granules and for normal amylose synthesis in Arabidopsis. PLOS Biol 2015;13(2):e1002080. doi:10.1371/journal.pbio.1002080
- ^ a b c d e Seung D. Amylose in starch: towards an understanding of biosynthesis, structure and function. New Phytol 2020;228:1490-1504. doi:10.1111/nph.16858
- ^ a b Jobling S. Improving starch for food and industrial applications. Curr Opin Plant Biol 2004;7(2):210-8. doi:10.1016/j.pbi.2003.12.001
- ^ Junejo S.A., Flanagan B.M., Zhang B., Dhital S. Starch structure and nutritional functionality – Past revelations and future prospects. Carbohydr Polym 2022;277:118837. doi:10.1016/j.carbpol.2021.118837
- ^ Funnell-Harris D.L., Sattler S.E., O’Neill P.M., Eskridge K.M., and Pedersen J.F. Effect of waxy (low amylose) on fungal infection of Sorghum grain. Phytopathology 2015;105(6):716-846. doi:10.1094/PHYTO-09-14-0255-R
- ^ a b c Wang J., Hu P., Chen Z., Liu Q., Wei C. Progress in high-amylose cereal crops through inactivation of starch branching enzymes. Front Plant Sci 2017;8:469. doi:10.3389/fpls.2017.00469
- ^ a b Li H-T., Zhang W., Zhu H, Chao C., Guo Q. Unlocking the potential of high-amylose starch for gut health: not all function the same. Fermentation 2023;9(2):134. doi:https://doi.org/10.3390/fermentation9020134
- ^ Bertoft E. Understanding starch structure: recent progress. Agronomy 2017;7:56. doi:10.3390/agronomy7030056
- ^ Hizukuri S., Takeda Y., Yasuda M., Suzuki A. Multi-branched nature of amylose and the action of debranching enzymes. Carbohydr Res 1981;94:205-213. doi:10.1016/S0008-6215(00)80718-1
- ^ Zhiguang C., Haixia Z., Min C., Fayong G., Jing L. The fine structure of starch: a review. NPJ Sci Food 2025;9(1):50. doi:10.1038/s41538-025-00414-x
- ^ a b Wang K., Hasjim J., Wu A.C., Henry R.J., Gilbert R.G. Variation in amylose fine structure of starches from different botanical sources. J Agric Food Chem 2014;62(19):4443-53. doi:10.1021/jf5011676
- ^ Teobaldi A.G., Carrillo Parra E.J., Barrera G.N., Ribotta P.D. The properties of damaged starch granules: the relationship between granule structure and water–starch polymer interactions. Foods 2025;14(1):21. doi:https://doi.org/10.3390/foods14010021
- ^ a b c Pfister B., Zeeman S.C. Formation of starch in plant cells. Cell Mol Life Sci 2016;73(14):2781-807. doi:10.1007/s00018-016-2250-x
- ^ Tetlow I.J., Emes M.J. A review of starch-branching enzymes and their role in amylopectin biosynthesis. IUBMB Life 2014;66(8):546-58. doi:10.1002/iub.1297
- ^ Szydlowski N., Ragel P., Raynaud S., Lucas M.M., Roldán I., Montero M., Muñoz F.J., Ovecka M., Bahaji A., Planchot V., Pozueta-Romero J., D’Hulst C., Mérida A. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 2009;21(8):2443-57. doi:10.1105/tpc.109.066522
- ^ a b Seung D., Boudet J., Monroe J., Schreier T.B., David L.C., Abt M., Lu K.J., Zanella M., Zeeman S.C. Homologs of PROTEIN TARGETING TO STARCH control starch granule initiation in Arabidopsis leaves. Plant Cell 2017;29(7):1657-1677. doi:10.1105/tpc.17.00222
- ^ Denyer K., Johnson P., Zeeman S., Smith A.M. The control of amylose synthesis. J Plant Physiol 2001;158: 479-487. doi:10.1078/0176-1617-00360
- ^ a b c Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ a b Heldt H-W. Plant biochemistry – 4th Edition. Elsevier Academic Press, 2010.
- ^ Leloir L.F., de Fekete M.A., Cardini C.E. Starch and oligosaccharide synthesis from uridine diphosphate glucose. J Biol Chem 1961;236:636-41. doi:10.1016/S0021-9258(18)64280-2
- ^ Vrinten P.L., Nakamura T. Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol 2000;122(1):255-64. doi:10.1104/pp.122.1.255
- ^ a b Gous P.W., Fox G.P. Review: amylopectin synthesis and hydrolysis – Understanding isoamylase and limit dextrinase and their impact on starch structure on barley (Hordeum vulgare) quality. Trends Food Sci Technol 2016;62:23-32. doi:10.1016/j.tifs.2016.11.013
- ^ Crofts N., Abe N., Oitome N.F., Matsushima R., Hayashi M., Tetlow I.J., Emes M.J., Nakamura Y., Fujita N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J Exp Bot 2015;66(15):4469-82. doi:10.1093/jxb/erv212
- ^ Kram A.M., Oostergetel G.T., Van Bruggen E. Localization of branching enzyme in potato tuber cells with the use of immunoelectron microscopy. Plant Physiol 1993;101(1):237-243. doi:10.1104/pp.101.1.237
- ^ a b Magallanes-Cruz P.A., Flores-Silva P.C., Bello-Perez L.A. Starch structure influences its digestibility: a review. J Food Sci 2017;82(9):2016-2023. doi:10.1111/1750-3841.13809
- ^ Birt D.F., Boylston T., Hendrich S., Jane J.L., Hollis J., Li L., McClelland J., Moore S., Phillips G.J., Rowling M., Schalinske K., Scott M.P., Whitley E.M. Resistant starch: promise for improving human health. Adv Nutr 2013;4(6):587-601. doi:10.3945/an.113.004325
- ^ Usuda H., Okamoto T., Wada K. Leaky gut: effect of dietary fiber and fats on microbiome and intestinal iarrier. Int J Mol Sci 2021;22(14):7613. doi:10.3390/ijms22147613
- ^ Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81(3):1031-64. doi:10.1152/physrev.2001.81.3.1031