Starch phosphorylase or alpha-glucan phosphorylase (EC 2.4.1.1) is a multimeric protein, with enzymatic and regulatory activity, that plays an important role in carbohydrate metabolism, both in prokaryotes and eukaryotes.[10]
The enzyme catalyzes the transfer of a glucosyl unit from glucose 1-phosphate to the non-reducing end of a nascent α-(1→4)-glucan, to which glucose is linked by an α-(1→4) glycosidic linkage.[6][8]The reaction is reversible and the direction depends on the phosphate/glucose-1-phosphate ratio present in vivo.[2]
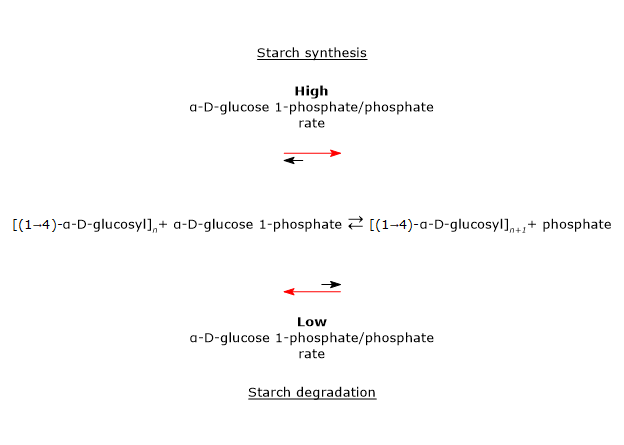
Note that, while starch synthase uses ADP-glucose as glucosyl donor, and glycogen synthase uses UDP-glucose, starch phosphorylase uses glucose 1-phosphate.[2]
Starch phosphorylase appears to be involved in both the synthesis and degradation of amylose and amylopectin.[9]
Industrially, the phosphorolytic action of starch phosphorylase is used in the production of glucose-1-phosphate and in the preparation of carbohydrates such as glucans and modified starches.[6]
Contents
Isoforms
At least two isoforms of starch phosphorylase are present in plants, one in the stroma of plastids, named Pho1, and Pho2, with cytosolic localization. Both isoforms play a critical role in the synthesis and degradation of starch.[6][8]
Starch degradation
Although alpha-amylase (EC 3.2.1.1) is the first enzyme to intervene in the polysaccharide degradation during the early stages of germination, and beta-amylase (EC 3.2.1.2) is the first enzyme to intervene in the transient starch degradation in chloroplasts, other enzyme activities have also been implicated, such as alpha-glucan water dikinase (EC 2.7.9.4) and phospho-glucan water dikinase (EC 2.7.9.5), and the debranching enzyme.[10]
Of the two isoenzymes of starch phosphorylase, Pho1 seems to have an indirect or regulatory action capable of influencing the activity of the other enzymes involved in starch degradation, while Pho2 is capable of degrading starch granules and other branched glucans.
Starch synthesis
During starch biosynthesis, starch phosphorylase, particularly Pho1, appears to perform both an enzymatic and regulatory action.
- Pho1 seems to be involved in the initial steps of starch synthesis, contributing to the elongation of the nascent chain.[1][8]
- Starch synthesis involves a set of at least five classes of enzymes, namely ADP-glucose pyrophosphorylase (EC 2.7.7.27), starch synthases, starch branching enzymes (EC 2.4.1.18), starch debranching enzymes (EC 3.2.1.41) and starch phosphorylases.[5][10] To these must be added proteins with no catalytic activity but needed for the correct synthesis of the starch granule. In the endosperm of cereals, the formation of multienzyme complexes between starch synthases and branching enzymes depends not only on specific phosphorylations of the proteins, but also on the presence of Pho1.[1][3]
- Starch phosphorylase is capable of forming a complex with disproportionating enzyme (EC 2.4.1.25).[1][10] Such complex seems capable of synthesizing short malto-oligosaccharides or MOS, which are α-(1→4)-glucans with a degree of polymerization between 2 and 7.[7] MOS act as primers for starch synthase IV and granule-bound starch synthase in the initial steps of amylopectin and amylose synthesis, respectively, a role which resembles that of glycogenin in glycogen synthesis.[4] MOS can also originate from the activity of starch debranching enzymes during the trimming of amylopectin molecules.
References
- ^ a b c Crofts N., Abe N., Oitome N.F., Matsushima R., Hayashi M., Tetlow I.J., Emes M.J., Nakamura Y., Fujita N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J Exp Bot 2015;66(15):4469-82. doi:10.1093/jxb/erv212
- ^ a b Orzechowski S. Starch metabolism in leaves. Acta Biochim Pol 2008;55(3):435-45. doi:10.18388/abp.2008_3049
- ^ Pareek V., Sha Z., He J., Wingreen N.S., Benkovic S.J. Metabolic channeling: predictions, deductions, and evidence. Mol Cell 2021;81(18):3775-3785. doi:10.1016/j.molcel.2021.08.030
- ^ Pfister B., Zeeman S.C., Rugen M.D., Field R.A., Ebenhöh O., Raguin A. Theoretical and experimental approaches to understand the biosynthesis of starch granules in a physiological context. Photosynth Res 2020;145:55-70. doi:10.1007/s11120-019-00704-y
- ^ Qu J., Xu S., Zhang Z., Chen G., Zhong Y., Liu L., Zhang R., Xue J., Guo D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci Rep 2018;8(1):12736. doi:10.1038/s41598-018-30411-y
- ^ a b c d Rathore R.S., Garg N., Garg S., Kumar A. Starch phosphorylase: role in starch metabolism and biotechnological applications. Crit Rev Biotechnol 2009;29(3):214-24. doi:10.1080/07388550902926063
- ^ Tetlow I.J., Bertoft E. A review of starch biosynthesis in relation to the building block-backbone model. Int J Mol Sci 2020;21(19):7011. doi:10.3390/ijms21197011
- ^ a b c Tickle P., Burrell M.M., Coates S.A., Emes M.J., Tetlow I.J., Bowsher C.G. Characterization of plastidial starch phosphorylase in Triticum aestivum L. endosperm. J Plant Physiol 2009;166(14):1465-78. doi:10.1016/j.jplph.2009.05.004
- ^ Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011
- ^ a b c d Yu G., Shoaib N., Xie Y., Liu L., Mughal N., Li Y., Huang H., Zhang N., Zhang J., Liu Y., Hu Y., Liu H., Huang Y. Comparative study of starch phosphorylase genes and encoded proteins in various Monocots and Dicots with emphasis on maize. Int J Mol Sci 2022;23:4518. doi:doi.org/10.3390/ijms23094518
