Pyruvic acid, an alpha-ketoacid, is a molecule with a central role in cellular metabolism.[1][2]
It can be produced through various metabolic pathways, mostly cytosolic, among which glycolysis is usually the most important pathway, while its fate depends on the cell type and the availability of oxygen, as it can be used for energy, biosynthetic, and anaplerotic purposes.[3]
Given its central role in cellular metabolism, mutations in the genes that encode the proteins involved in its metabolism cause, in humans, mild to severe diseases.[4]
Contents
Chemical properties
Pyruvic acid or, according to the IUPAC nomenclature, 2-oxopropanoic acid, has a molecular formula C3H4O3, condensed formula CH3COCOOH, and molecular weight of 88.06 g/mol.
It belongs to the group of alpha-ketoacids, meaning keto acids that have the carbonyl group adjacent to the carboxylic acid. It has the simplest chemical structure among the alpha-ketoacids.[1]
In purified form it appears as a colorless liquid, with a smell similar to that of acetic acid.
Its acid dissociation constant (pKa), at 25 °C, is equal to 2.45. It is therefore a very strong acid, and, at physiological pH, both in cells and in extracellular fluids, is present almost entirely in its anionic form, pyruvate.
Its melting point is 13.8 °C (56.84 °F; 286.95 K), while its boiling point is 163.5 °C (329 °F; 438 K).[1]
Pyruvate metabolism
The biosynthetic pathways leading to pyruvic acid production, as well as its subsequent utilization, depend on the cell type and/or the availability of oxygen.[3]
In cells with mitochondria, pyruvate metabolism consists of a cytosolic and a mitochondrial phase.
In the cytosol, several metabolic pathways lead to its formation, namely, glycolysis, the oxidation of lactate, the reaction catalyzed by the cytosolic malic enzyme, and the catabolism of at least six amino acids, the most important of which is alanine.
Pyruvic acid production from alanine and lactate can also occur in the mitochondrial matrix.[5]
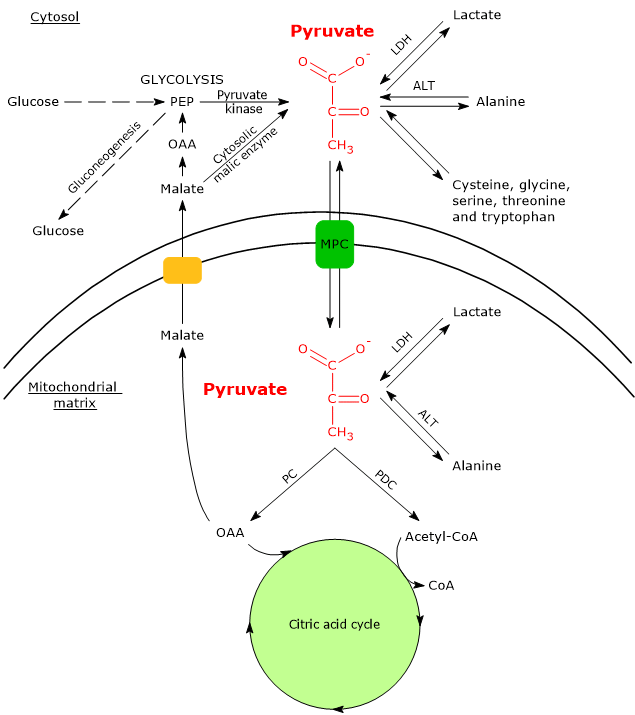 The pyruvic acid produced in the cytosol, by means of specific transporters, enters the mitochondrion where it can be used for energy, entering the citric acid cycle, and/or biosynthetic/anaplerotic purposes, depending on the needs of the cell.[6]
The pyruvic acid produced in the cytosol, by means of specific transporters, enters the mitochondrion where it can be used for energy, entering the citric acid cycle, and/or biosynthetic/anaplerotic purposes, depending on the needs of the cell.[6]
If, instead, we consider cells without mitochondria, such as red blood cells, and, under hypoxic conditions, cells with mitochondria too, pyruvate is reduced to lactate and/or leaves the cell as such to be metabolized in other tissues, for example cardiac muscle.[7]
Glycolysis
Under physiological conditions, in most cells pyruvic acid is mainly derived from glycolysis, of which it is one of the three products, with ATP and NADH.
In the last step of the glycolytic pathway, pyruvate kinase (EC 2.7.1.40) catalyzes a substrate-level phosphorylation that leads to the transfer of the phosphoryl group from phosphoenolpyruvate to ADP. The reaction, essentially irreversible, produces one molecule of ATP and one of pyruvate.
Phosphoenolpyruvate + ADP + H+ → Pyruvate + ATP
Therefore, during the glycolytic pathway, two molecules of pyruvate are produced from a glucose molecule.[2][8]
Lactate dehydrogenase
In the cytosol, pyruvic acid can be produced from lactic acid.
The enzyme lactate dehydrogenase (LDH) (EC 1.1.1.27) catalyzes the reversible conversion of pyruvate to lactate and of NADH to NAD+.
Pyruvate + NADH + H+ ⇄ Lactate + NAD+
The direction of the reaction depends on lactate dehydrogenase isozymes present and the NADH/NAD+ ratio in the cytosol.[3][5]
Isoenzymes in which the H subunit predominates, such as LDH-1, predominate in cardiac muscle, an exclusively aerobic tissue, where they catalyze the oxidation of lactate (formed in other tissues) to pyruvate, which is then used for energy.
The oxidation of lactate produced for example by red blood cells or skeletal muscle under hypoxic conditions, can also occur in hepatocytes, favored by the low NADH/NAD+ ratio in the cytosol, although LDH-5 is the main isoenzyme. In the hepatocyte, pyruvic acid can be enter gluconeogenesis, which in this case is part of the Cori cycle, or be oxidized for energy.[9]
Conversely, in skeletal muscle fiber under hypoxic conditions, in which the pyruvate dehydrogenase complex is inhibited and oxidative phosphorylation is blocked, in order for glycolysis to proceed pyruvate is reduced to lactate with the concomitant oxidation of NADH to NAD+.[10] Note that the conversion of glucose into lactate is defined as lactic fermentation. This reduction is favored by the fact that in skeletal muscle fiber isoenzymes with a prevalence of the M subunit predominate, such as LDH-4 and LDH-5.[11]
Alanine aminotransferase
Another source of pyruvic acid is alanine, an amino acid particularly abundant in muscle proteins.
The utilization of the carbon skeleton of amino acids for energy and/or anabolic purposes involves the removal of the basic amino group, which occurs in reactions catalyzed by enzymes called transaminases (EC 2.6.1-), and the subsequent disposal of nitrogen in a non-toxic form through the urea cycle.[2][8]
The removal of the amino group of alanine is catalyzed by alanine aminotransferase (ALT; EC 2.6.1.2). The reaction, reversible, leads to the formation of pyruvate and glutamate.
Alanine + alpha-Ketoglutarate ⇄ Pyruvate + Glutamate
Two forms of ALT have been identified: ALT1, localized in the cytosol, and ALT2, localized in the mitochondrial matrix.[4] Hence, pyruvic acid can be produced by transamination of alanine also in the mitochondrial matrix.
Alanine is one of the main gluconeogenic precursors, and, through the glucose-alanine cycle, represents a link between the metabolism of carbohydrates and amino acids.[12]
In the cytosol, the carbon skeletons of five other amino acids, namely cysteine, glycine, serine, threonine and tryptophan, can be converted partly or entirely to pyruvate.[2]
Malic enzyme
Pyruvate can also be produced from malate.
In the reaction catalyzed by the cytosolic malic enzyme (EC 1.1.1.40), malate undergoes an oxidative decarboxylation to yield pyruvate.[3]
Malate + NADP+ → Pyruvate + CO2 + NADPH + H+
Malic enzyme plays an important role in the transport of intermediates of the citric acid cycle, such as, in addition to malate, oxaloacetate and citrate, between the cytosol and the mitochondrial matrix.[7]
Mitochondrial pyruvate carriers
In cells with mitochondria, most of the pyruvate produced in the cytosol enters the mitochondrial matrix passing through the outer and the inner mitochondrial membranes. In the mitochondrial matrix pyruvate can then be used for both anabolic and catabolic purposes.
The passage through the outer mitochondrial membrane occurs by free diffusion through non-specific voltage-dependent anion channels (VDACs) or porins, the most abundant proteins of the outer mitochondrial membrane, whose function is to mediate the exchange of ions and small molecules, including, in addition to pyruvate, also ATP, NADH and others, between the cytosol and the intermembrane space of mitochondria.[13][14]
Conversely, the inner mitochondrial membrane is impermeable to charged molecules, which allows the maintenance of the proton gradient needed for oxidative phosphorylation to occur. The passage of pyruvate therefore occurs through specific transporters, the mitochondrial pyruvate carriers (MPC), a hetero-oligomeric complex of two subunits, indicated as MPC1 and MPC2. This transport is coupled to a symport of protons. MPC therefore links the cytosolic and mitochondrial metabolism of pyruvate.[15][16]
Pyruvate dehydrogenase complex
Once in the mitochondrial matrix, pyruvate is mostly oxidized to carbon dioxide in order to support ATP production.
The first step in this oxidation process involves the pyruvate dehydrogenase complex (PDC), one of the most important multienzyme complexes present in cells. The complex catalyzes the irreversible oxidative decarboxylation of pyruvate to form acetyl-coenzyme A (acetyl-CoA) and a carbon dioxide molecule, with the release of two electrons, which are carried by NAD.
Pyruvate + CoA + NAD+ → Acetyl-CoA + NADH + H+ + CO2
The acetyl group can enter the citric acid cycle to be completely oxidized to carbon dioxide, with the production of a molecule of GTP, 3 molecules of NADH, one of FADH2. The two reduced coenzymes, through oxidative phosphorylation, will allow the production of further ATP molecules.[2]
Alternatively, the acetyl group derived from pyruvic acid can be used for anabolic purposes, among which the synthesis of some lipids, such as of fatty acids, phospholipids and cholesterol, or for regulatory purposes through histone acetylation.
Since lipid biosynthesis occurs in the cytosol and histone acetylation in the nucleus, acetyl-CoA must leave the mitochondrial matrix, a process that requires the formation of citrate through the condensation between the acetyl group and carbonyl of oxaloacetate, reaction catalyzed by citrate synthase (EC 2.3.3.1).
Once citrate has reached the cytosol, through a citrate carrier, an integral protein of the inner mitochondrial membrane, it is cleaved to acetyl-CoA and oxaloacetate in the reaction catalyzed by citrate lyase (EC 4.1.3.6).[17]
Pyruvate carboxylase
In the mitochondrial matrix, pyruvic acid can be carboxylated to oxaloacetate in an irreversible reaction catalyzed by pyruvate carboxylase (PC) (EC 6.4.1.1).[8]
Pyruvate + HCO3– + ATP ⇄ Oxaloacetate + ADP + Pi
Several intermediates of the citric acid cycle are precursors for the synthesis of various molecules. It follows that each of these intermediates removed from the citric acid cycle must be reintegrated for the cycle to continue. Reactions that reintegrate the cycle are defined as anaplerotic. In this perspective, the reaction catalyzed by pyruvate carboxylase plays an anaplerotic function, catalyzing the formation of oxaloacetate.[6]
References
- ^ a b c National Center for Biotechnology Information. PubChem Compound Summary for CID 1060, Pyruvic Acid. https://pubchem.ncbi.nlm.nih.gov/compound/Pyruvic-Acid. Accessed Aug. 19, 2024
- ^ a b c d e Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ a b c d Berg J.M., Tymoczko J.L., Gregory J.G. Jr, Stryer L. Biochemistry. 9th Edition. W.H. Freeman and Company, 2019.
- ^ a b Gray L.R., Tompkins S.C., Taylor E.B. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 2014;71(14):2577-604. doi:10.1007/s00018-013-1539-2
- ^ a b Markert C.L., Shaklee J.B., Whitt G.S. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science 1975;189(4197):102-14. doi:10.1126/science.1138367
- ^ a b Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 2002;277(34):30409-12. doi:10.1074/jbc.R200006200
- ^ a b McCommis K.S. and Finck B.N. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem J 2015;466(3):443-454. doi:10.1042/BJ20141171
- ^ a b c Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012.
- ^ Gleeson T.T. Post-exercise lactate metabolism: a comparative review of sites, pathways, and regulation. Annu Rev Physiol 1996;58:565-81. doi:10.1146/annurev.ph.58.030196.003025
- ^ Li X., Yang Y., Zhang B., Lin X., Fu X., An Y., Zou Y., Wang J.X., Wang Z., Yu T. Lactate metabolism in human health and disease. Signal Transduct Target Ther 2022;7(1):305. doi:10.1038/s41392-022-01151-3
- ^ Farhana A., Lappin S.L. Biochemistry, lactate dehydrogenase. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557536/
- ^ Felig P., Pozefsk T., Marlis E., Cahill G.F. Alanine: key role in gluconeogenesis. Science 1970;167(3920):1003-1004. doi:10.1126/science.167.3920.1003
- ^ Colombini M. The VDAC channel: molecular basis for selectivity. Biochim Biophys Acta 2016;1863(10):2498-502. doi:10.1016/j.bbamcr.2016.01.019
- ^ Young M.J., Bay D.C., Hausner G., Court D.A. The evolutionary history of mitochondrial porins. BMC Evol Biol 2007;7:31. doi:10.1186/1471-2148-7-31
- ^ Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.C., Cox J.E., Cardon C.M., Van Vranken J.G., Dephoure N., Redin C., Boudina S., Gygi S.P., Brivet M., Thummel C.S., Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012;337(6090):96-100. doi:10.1126/science.1218099
- ^ Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B., Kunji E.R., Martinou J.C. Identification and functional expression of the mitochondrial pyruvate carrier. Science 2012;337(6090):93-6. doi:10.1126/science.1218530
- ^ Zara V., Assalve G., Ferramosca A. Multiple roles played by the mitochondrial citrate carrier in cellular metabolism and physiology. Cell Mol Life Sci 2022;79(8):428. doi:10.1007/s00018-022-04466-0