Glucogenic amino acids are defined as amino acids whose carbon skeletons can be fully or partially catabolized into precursors for glucose synthesis or gluconeogenesis, hence the name.[1]
Amino acids are the building blocks of proteins. When their pool meets the cellular needs for protein synthesis, since there are no amino acid stores in the body comparable to glycogen for glucose or triglycerides for fatty acids, the excess amino acids are catabolized into precursors of the citric acid cycle and used for energy production.[2]
However, when the energy needs of the cell are met, the carbon skeletons resulting from amino acid catabolism are used for the synthesis of fatty acids, ketone bodies, or glucose.[3]
Glucogenic and ketogenic amino acids are classified based on the metabolic fate of their carbon skeletons. However, this classification is not clear-cut because, among the twenty standard amino acids found in proteins, five are both glucogenic and ketogenic.[4]
Glucose synthesis from glucogenic amino acids is possible because their carbon skeletons are fully or partially catabolized into pyruvate, oxaloacetate, α-ketoglutarate, succinyl-CoA, or fumarate.[5]
Pyruvate and oxaloacetate are intermediates of gluconeogenesis, while the entry of the other three metabolites into the citric acid cycle results in a net gain of carbon units, which can subsequently enter gluconeogenesis as oxaloacetate.[6]
Glucogenic amino acids play a crucial role during prolonged fasting, serving as a major source of precursors for gluconeogenesis, second only to glycerol derived from adipose tissue lipolysis.[7]
Contents
- What are glucogenic amino acids?
- Biochemical basis
- Glucogenic amino acids during prolonged fasting
- References
What are glucogenic amino acids?
Of the twenty amino acids that make up proteins, thirteen are exclusively glucogenic, meaning that the catabolism of their carbon skeletons yields only glucose precursors.[4]
| Amino acid | Catabolite | ||
| ketogenic | glucogenic | ||
| Alanine | Pyruvate | ||
| Arginine | α-Ketoglutarate | ||
| Asparagine | Fumarate | ||
| Aspartate | Oxaloacetate | ||
| Cysteine | Pyruvate | ||
| Phenylalanine * | Acetoacetyl-CoA | Fumarate | |
| Glycine | Pyruvate | ||
| Glutamate | α-Ketoglutarate | ||
| Glutamine | α-Ketoglutarate | ||
| Isoleucine * | Acetyl-CoA | Succinyl-CoA | |
| Histidine | α-Ketoglutarate | ||
| Methionine | Succinyl-CoA | ||
| Proline | α-Ketoglutarate | ||
| Serine | Pyruvate | ||
| Tirosine * | Acetoacetyl-CoA | Fumarate | |
| Threonine * | Acetyl-CoA | Succinyl-CoA | |
| Tryptophan * | Acetyl-CoA | Acetoacetyl-CoA | Pyruvate |
| Valine | Succinyl-CoA | ||
| * Amino acids that are both glucogenic and ketogenic. | |||
Five amino acids, namely isoleucine, phenylalanine, threonine, tryptophan, and tyrosine, are both glucogenic and ketogenic, as a portion of their carbon skeleton is catabolized into glucogenic precursors, while another portion is converted into acetyl-CoA and/or acetoacetyl-CoA.[8]
Before amino acid carbon skeletons can be utilized, their amino groups must first be removed. Alanine and glutamine, the primary molecules responsible for transporting amino groups from extrahepatic tissues to the liver, are particularly important glucogenic amino acids in mammals. Alanine is the primary gluconeogenic substrate for the liver, reaching it from muscle and other peripheral tissues via the glucose-alanine cycle.[9]
Biochemical basis
As with ketogenic amino acids, analyzing the stoichiometry of the citric acid cycle clarifies why the carbon skeleton of glucogenic amino acids acts as a precursor for glucose synthesis. The key factor is where these carbons enter the citric acid cycle.[6]
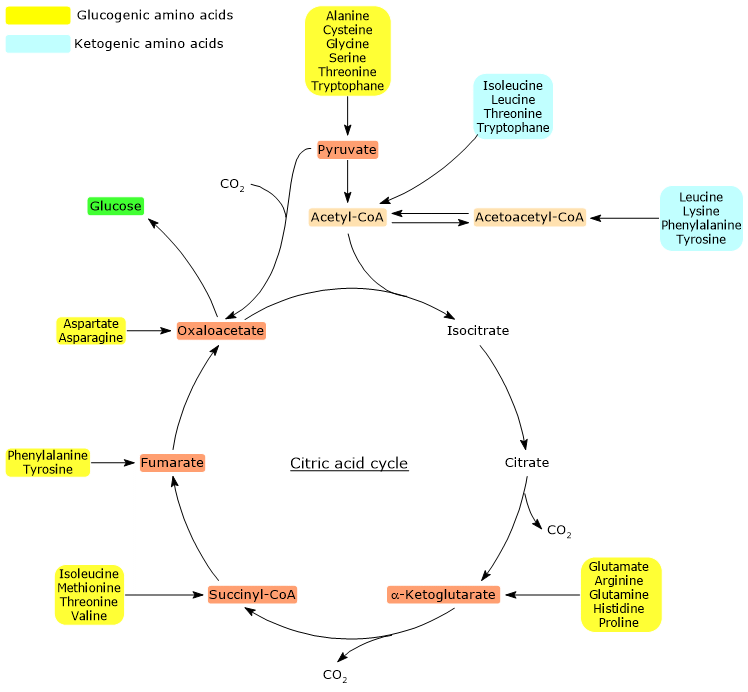 When carbons derived from amino acids enter the cycle as α-ketoglutarate, succinyl-CoA, or fumarate, they result in a net gain of carbon units. Except for α-ketoglutarate, the other metabolites enter the cycle downstream of the two oxidative decarboxylation reactions catalyzed by isocitrate dehydrogenase (EC 1.1.1.42) and the α-ketoglutarate dehydrogenase multienzyme complex.[5]
When carbons derived from amino acids enter the cycle as α-ketoglutarate, succinyl-CoA, or fumarate, they result in a net gain of carbon units. Except for α-ketoglutarate, the other metabolites enter the cycle downstream of the two oxidative decarboxylation reactions catalyzed by isocitrate dehydrogenase (EC 1.1.1.42) and the α-ketoglutarate dehydrogenase multienzyme complex.[5]
This leads to a net gain of one carbon unit if entry occurs at α-ketoglutarate, or two if it occurs at succinyl-CoA or fumarate. These carbon units can subsequently enter gluconeogenesis as oxaloacetate.[4]
Furthermore, because the reaction catalyzed by the pyruvate dehydrogenase complex, i.e., the oxidative decarboxylation of pyruvate to acetyl-CoA, is irreversible, and no alternative metabolic pathways exist in animals to synthesize pyruvate from acetyl-CoA, acetyl-CoA cannot serve as a glucogenic substrate.[6]
Glucogenic amino acids during prolonged fasting
Glucogenic amino acids play a crucial role during prolonged fasting and in diets with severe carbohydrate restriction. Under these conditions, they are among the primary precursors for glucose synthesis.[6] The utilization of their carbon skeletons, along with glycerol and propionate, a short-chain fatty acid, helps maintain glycemic homeostasis via gluconeogenesis when liver glycogen stores are depleted.[7]
However, even under physiological conditions, such as during cellular protein turnover or after a protein-rich meal, amino acids exceeding the requirements for protein synthesis are catabolized. Depending on the metabolic state and the specific amino acid, they may be used for energy production or anabolic processes, including glucose synthesis. The glucose produced can subsequently be utilized for glycogen synthesis, converted into ketone bodies or fatty acids.[2]
References
- ^ D’Andrea G. Classifying amino acids as gluco(glyco)genic, ketogenic, or both. Biochem Educ 2000;28(1):27-28. doi:10.1016/s0307-4412(98)00271-4
- ^ a b Brosnan J.T. Interorgan amino acid transport and its regulation. J Nutr 2003;133(6 Suppl 1):2068S-2072S. doi:10.1093/jn/133.6.2068S
- ^ Litwack G. Human biochemistry. 2nd Edition. Academic Pr, 2021.
- ^ a b c Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012.
- ^ a b Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012.
- ^ a b c d Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011.
- ^ a b Kuriyama H., Shimomura I., Kishida K., Kondo H., Furuyama N., Nishizawa H., Maeda N., Matsuda M., Nagaretani H., Kihara S., Nakamura T., Tochino Y., Funahashi T., Matsuzawa Y. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 2002;51(10):2915-2921. 10.2337/diabetes.51.10.2915
- ^ Rosenthal M.D., Glew R.H. Medical Biochemistry – Human Metabolism in Health and Disease. John Wiley J. & Sons, Inc., 2009.
- ^ Felig P., Pozefsk T., Marlis E., Cahill G.F. Alanine: key role in gluconeogenesis. Science 1970;167(3920):1003-1004. doi:10.1126/science.167.3920.1003