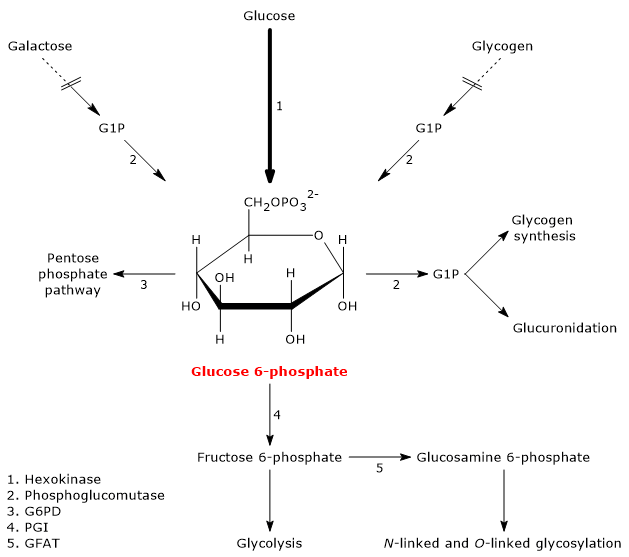
Glucose 6-phosphate (G6P) is a key metabolite in carbohydrate metabolism and, more broadly, in cellular metabolism.[1]
In animal and bacterial cells, it mainly derives from the reaction catalyzed by the enzyme hexokinase, which catalyzes the first step of the glycolytic pathway. Alternatively, it can be produced through glycogenolysis, a catabolic process in which glycogen is degraded to provide glucose in response to specific metabolic needs.[2] A third, although quantitatively less significant, biosynthesis pathway involves the conversion of galactose via the Leloir pathway.[3]
Glucose 6-phosphate represents a crucial cellular metabolic hub. Depending on the cell’s needs, it can proceed through glycolysis, be diverted into the hexose monophosphate shunt, or be used for glycogen synthesis and macromolecular glycosylation.[4] Furthermore, in the liver, it can be converted into free glucose or participate in detoxification processes via glucuronidation.[5]
Contents
- Chemical properties
- How is glucose 6-phosphate formed?
- Why is the phosphorylation of glucose to glucose 6-phosphate important?
- Metabolic fate of glucose 6-phosphate
- References
Chemical properties
Glucose 6-phosphate has a molecular weight of 260.14 g/mol and a molecular formula of C6H13O9P.
According to IUPAC nomenclature, its systematic name is [(2R,3R,4S,5R)-2,3,4,5-tetrahydroxy-6-oxohexyl] dihydrogen phosphate.
Its pKa1 and pKa2, at 25°C are 1.65 and 6.11, respectively. Therefore, at physiological pH, it exists almost exclusively in its ionized form and carries a charge of −2.[6]
How is glucose 6-phosphate formed?
In animal and bacterial cells (excluding photosynthesis), glucose 6-phosphate is primarily produced through two pathways: the direct phosphorylation of glucose and, in animal cells, glycogenolysis. It can also be synthesized from galactose.[2]
Hexokinase
Once glucose enters the cell, its main metabolic fate is to be phosphorylated to glucose 6-phosphate.
This reaction is the first of the ten steps of glycolysis and also the first of the two phosphorylation steps that the monosaccharide undergoes during this metabolic pathway.
Hexokinase (EC 2.7.1.1), an enzyme found in the cells of all organisms and present in four isoforms in humans, catalyzes this irreversible reaction, phosphorylating glucose at the C-6 position using one ATP molecule.
Glucose + ATP → Glucose 6-phosphate + ADP + H⁺
This glucose 6-phosphate synthesis pathway is particularly important when the monosaccharide is available from external sources, such as after a meal.[1]
Glycogenolysis
Glucose 6-phosphate can also be derived from glycogenolysis, the metabolic pathway by which glucose molecules stored in glycogen, a polysaccharide, are released when needed.
During fasting — for example, at night or between meals — but also during physical activity, glycogenolysis, through the intervention of three enzymatic activities, namely, glycogen phosphorylase (EC 2.4.1.1), alpha-(1,4)-glucan-6-glycosyltransferase (EC 2.4.1.25), and amylo-alpha-(1,6)-glucosidase, or debranching enzyme (EC 3.2.1.33), leads to the release of glucose: approximately 90 percent in the form of glucose 1-phosphate (G1P), and the remaining 10 percent directly as glucose.
Glucose 1-phosphate is then isomerized to glucose 6-phosphate in the reversible reaction catalyzed by phosphoglucomutase (EC 5.4.2.2).[2]
Galactose
Galactose is a monosaccharide present in milk and dairy products, where it is mainly found as a component, along with glucose, of lactose. Through the Leloir pathway, it can be converted into glucose 1-phosphate, which in turn can be isomerized to glucose 6-phosphate.[7]
The Leloir pathway allows the formation of metabolites that can enter various metabolic routes, both anabolic and catabolic, depending on the tissue and the metabolic conditions of the cell. Among the possible fates of galactose along this pathway is its conversion into glucose 1-phosphate, which can then be isomerized to glucose 6-phosphate.[3]
Why is the phosphorylation of glucose to glucose 6-phosphate important?
The phosphorylation of glucose to glucose 6-phosphate is metabolically important for several reasons.
- Glucose is a neutral molecule whose passage through the plasma membrane occurs via facilitated diffusion, mediated by specific protein transporters belonging to the GLUT or SLC2A family of membrane transport proteins.[8]
Phosphorylation at carbon-6 (C-6) gives glucose a negative charge, preventing it from crossing the membrane, partly because of the absence of specific transporters for phosphorylated sugars. Therefore, phosphorylation traps the monosaccharide inside the cell.[4] - Thanks to the phosphorylation at C-6 and the lack of transporters for phosphorylated sugars, it is no longer necessary to expend energy to retain glucose 6-phosphate inside the cell, despite the large difference between its intracellular and extracellular concentrations.[9]
- The rapid conversion of glucose into glucose 6-phosphate also helps maintain a low intracellular concentration of free glucose, thereby facilitating its entry by facilitated diffusion.[4]
- The addition of a phosphate group increases the energy content of glucose, that is, it begins to destabilize the molecule, facilitating its subsequent metabolism.[9]
Metabolic fate of glucose 6-phosphate
Glucose 6-phosphate is a central hub in carbohydrate metabolism. As a common intermediate in various metabolic pathways, its fate depends on the metabolic needs of the cell. It can participate in several metabolic route, both anabolic and catabolic, such as glycolysis, gluconeogenesis, the pentose phosphate pathway, glycogen synthesis, and hexosamine pathway. Finally, it is also involved in detoxification processes through the glucuronidation of molecules destined for excretion.[10]
Glycolysis
Glycolysis is a fundamental metabolic pathway, not only for energy production but also as a source of intermediates for other metabolic pathways.[11]
Glucose 6-phosphate can continue along the pathway, becoming a substrate for the reaction catalyzed by phosphoglucose isomerase (PGI; EC 5.3.1.9). This enzyme catalyzes its reversible isomerization to fructose 6-phosphate, an example of functional group isomerism. However, fructose 6-phosphate is not an exclusive metabolite of glycolysis.
In the third step of the glycolytic pathway, fructose 6-phosphate undergoes a second phosphorylation to form fructose 1,6-bisphosphate. This reaction, catalyzed by phosphofructokinase-1 (PFK-1; EC 2.7.1.11), is irreversible and leads to the formation of a metabolite that is exclusive to glycolysis — and, in the reverse direction, to gluconeogenesis.[9]
Hexosamine pathway
Fructose 6-phosphate, produced by the isomerization of glucose 6-phosphate, is one of the key substrates of the hexosamine pathway. This metabolic pathway accounts for approximately 2–5 percent of glucose metabolism and is essential for the glycosylation of macromolecules.[10]
In the first step, fructose 6-phosphate is converted to glucosamine 6-phosphate in a reaction catalyzed by the enzyme glutamine:fructose-6-phosphate aminotransferase (GFAT; EC 2.6.1.16), which catalyzes the first committed step of the route. In this reaction, the amino acid glutamine serves as the donor of the amino group.[12]
The subsequent steps ultimately lead to the synthesis of UDP-N-acetylglucosamine (UDP-GlcNAc), which is used in both N-linked and O-linked glycosylation of proteins, lipids, and nucleic acids.[13]
Pentose phosphate pathway
Glucose 6-phosphate can enter the pentose phosphate pathway, also known as the hexose monophosphate shunt, an alternative to glycolysis for the oxidative metabolism of glucose.[14]
Entry into the pathway occurs at the level of the reaction catalyzed by glucose 6-phosphate dehydrogenase (G6PD; EC 1.1.1.49). In this reaction, G6P is oxidized to 6-phosphoglucono-δ-lactone, which then continues along the metabolic pathway.
The pentose phosphate pathway serves a dual function: the synthesis, in variable ratios depending on the metabolic conditions of the cell, of NADPH and ribose-5-phosphate.
- NADPH is a reduced coenzyme essential for biosynthetic reactions such as the synthesis of fatty acids and cholesterol, as well as for antioxidant defense by reducing oxidized glutathione.[1]
- Ribose 5-phosphate, a five-carbon sugar, is essential for the synthesis of nucleotides and nucleic acids, and is therefore particularly important during periods of growth.[4]
It is estimated that over 10% of metabolized glucose is channeled through this pathway, which, interestingly, while oxidizing the monosaccharide, neither produces nor directly consumes ATP.[15]
Glycogen synthesis
Glucose 6-phosphate can be used to replenish cellular glycogen reserves. The anabolic pathway leading to polysaccharide biosynthesis occurs when carbohydrates are available, glucose demand is low, and there is an energy surplus. Glycogen synthesis is particularly important in the liver and muscle.[16]
During glycogen synthesis, glucose 6-phosphate is isomerized to glucose 1-phosphate in a reaction catalyzed by phosphoglucomutase. This enzyme also functions in glycogenolysis, catalyzing the reverse reaction. The direction of the reaction is determined by the relative concentrations of the two sugar phosphates.
In the next step, the carbon skeleton is activated by the transfer of a UDP group in a reaction catalyzed by UDP-glucose pyrophosphorylase (EC 2.7.7.9).
The resulting UDP-glucose then serves as the substrate for the reaction catalyzed by glycogen synthase (EC 2.4.1.11) and is used to elongate glycogen chains. UDP-glucose is also used as a sugar donor in the synthesis of glycolipids.[4]
Detoxification processes
Detoxification processes enable the elimination of xenobiotics, drugs, and catabolic by-products such as bilirubin. These metabolic pathways occur primarily in the liver, where glucose 6-phosphate acts as a precursor in the synthesis of glucuronic acid.[17]
G6P, similar to its role in glycogen synthesis, is first activated to UDP-glucose. UDP-glucose is then oxidized to UDP-glucuronate in a reaction catalyzed by UDP-glucose 6-dehydrogenase (EC 1.1.1.22). UDP-glucuronate serves as a glucuronic acid donor in reactions catalyzed by UDP-glucuronosyltransferase (EC 2.4.1.17). This process, known as glucuronidation, increases the hydrophilicity of otherwise lipophilic compounds, thereby facilitating their elimination via bile or urine.[5]
References
- ^ a b c Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012.
- ^ a b c Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011.
- ^ a b Conte F., van Buuringen N., Voermans N.C., Lefeber D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: time for a closer look. Biochim Biophys Acta Gen Subj 2021;1865(8):129898. doi:10.1016/j.bbagen.2021.129898
- ^ a b c d e Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010.
- ^ a b Rowland A., Miners J.O., Mackenzie P.I. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol 2013;45(6):1121-32. doi:10.1016/j.biocel.2013.02.019
- ^ National Center for Biotechnology Information. PubChem Compound Summary for CID 439958, Glucose-6-Phosphate. https://pubchem.ncbi.nlm.nih.gov/compound/D-Glucose-6-phosphate. Accessed Apr. 19, 2025.
- ^ Leloir L.F., de Fekete M.A., Cardini C.E. Starch and oligosaccharide synthesis from uridine diphosphate glucose. J Biol Chem 1961;236:636-41. doi:10.1016/S0021-9258(18)64280-2
- ^ Thorens B., Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 2010;298(2):E141-5. doi:10.1152/ajpendo.00712.2009
- ^ a b c Berg J.M., Tymoczko J.L., and Stryer L. Biochemistry. 5th Edition. W. H. Freeman and Company, 2002.
- ^ a b Rajas F., Gautier-Stein A., Mithieux G. Glucose-6 phosphate, a central hub for liver carbohydrate metabolism. Metabolites 2019;9(12):282. doi:10.3390/metabo9120282
- ^ Kierans S.J., Taylor C.T. Glycolysis: a multifaceted metabolic pathway and signaling hub. J Biol Chem 2024;300(11):107906. doi:10.1016/j.jbc.2024.107906
- ^ Milewski S. Glucosamine-6-phosphate synthase—the multi-facets enzyme. Biochim Biophys Acta 2002;1597(2):173-92. doi:10.1016/s0167-4838(02)00318-7
- ^ Paneque A., Fortus H., Zheng J., Werlen G., Jacinto E. The hexosamine biosynthesis pathway: regulation and function. Genes (Basel) 2023;14(4):933. doi:10.3390/genes14040933
- ^ Horecker B.L. The pentose phosphate pathway. J Biol Chem 2002;277(50):47965-47971. doi:10.1074/jbc.X200007200
- ^ Michal G., Schomburg D. Biochemical pathways. An atlas of biochemistry and molecular biology. 2nd Edition. John Wiley J. & Sons, Inc. 2012.
- ^ Adeva-Andany M.M., González-Lucán M., Donapetry-García C., Fernández-Fernández C., Ameneiros-Rodríguez E. Glycogen metabolism in humans. BBA Clin 2016;5:85-100. doi:10.1016/j.bbacli.2016.02.001
- ^ Yang G., Ge S., Singh R., Basu S., Shatzer K., Zen M., Liu J., Tu Y., Zhang C., Wei J., Shi J., Zhu L., Liu Z., Wang Y., Gao S., Hu M. Glucuronidation: driving factors and their impact on glucuronide disposition. Drug Metab Rev 2017;49(2):105-138. doi:10.1080/03602532.2017.1293682