Anthocyanins are a subgroup of flavonoids, therefore they are polyphenols, which give plants their distinctive colors.
They are water soluble pigments and are present in the vacuolar sap of the epidermal tissues of flowers and fruit. They are responsible for the colors of the most of the petals, fruits and vegetables, and of some varieties of cereals such as black rice. In fact, they impart red, pink and purple to blue colors to berries, red apples, red grapes, cherries, and of many other fruits, red lettuce, red cabbage, onions or eggplant, but also red wine.
Together with carotenoids, they are responsible for autumn leaf color and used commercially as food colours.
Finally, anthocyanins contribute to attract animals when a fruit is ready to eat or a flower is ready for pollination.
They are bioactive compounds found in plant foods that have a double interest for man:
- the first one, a technological interest, due to their effects on the organoleptic characteristics of food products;
- the other due to their healthy properties, being implicated in the protection against cardiovascular risk.
In fact:
in vitro, they have an antioxidant activity, due to their ability to delocalize electrons and form resonance structures, and a protective role against oxidation of low density lipoproteins (LDL);
like other polyphenols, such as catechins, proanthocyanidins and other uncolored flavonoids, they can regulate different signaling pathways involved in cell growth, differentiation and survival.
Contents
Chemical structure
The basic chemical structure is flavylium cation (2-phenyl-1-benzopyrilium), which links hydroxyl (-OH) and/or methoxyl (-OCH3) groups, and one or more sugars.
The sugar-free molecule is called anthocyanidins.
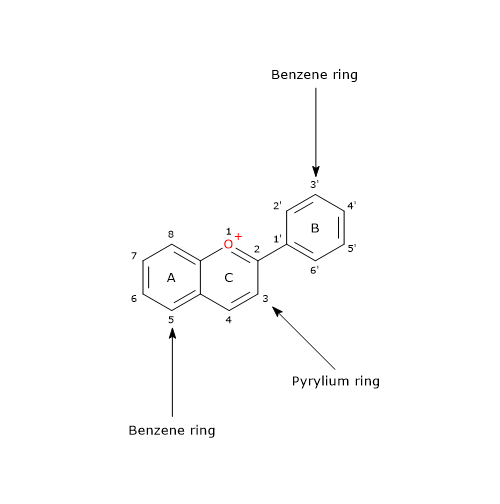
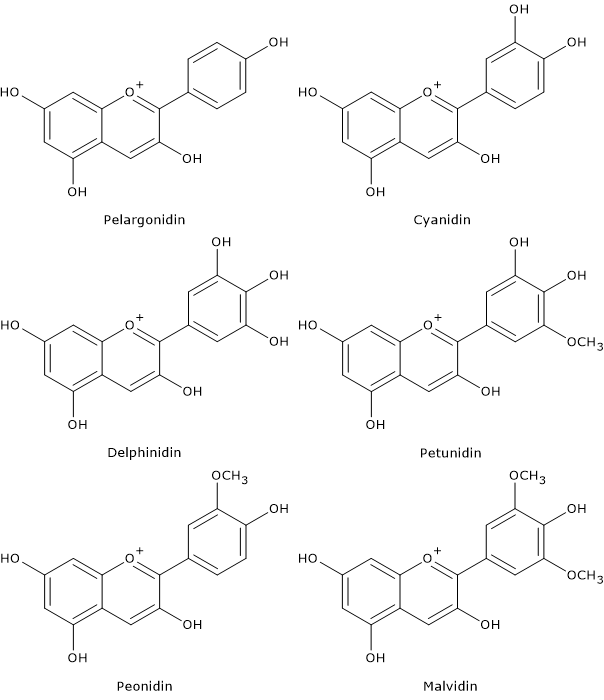
Depending on the number and position of hydroxyl and methoxyl groups, various anthocyanidins have been described, and of these, six are commonly found in vegetables and fruits:
- pelargonidin
- cyaniding
- delphinidin
- petunidin
- peonidin
- malvidin
Anthocyanins, as most of the other flavonoids, are present in plants and plant foods in the form of glycosides, that is, linked to one or more sugar units.
The most common carbohydrates present in these natural pigments are:
The sugars are linked mainly to the C3 position as 3-monoglycosides, to the C3 and C5 positions as diglycosides, with the possible forms: 3-diglycosides, 3,5-diglycosides, and 3-diglycoside-5-monoglycosides. Glycosylations have been also found at C7, C3′ and C5′ positions.
The structure of these molecules is further complicated by the bond to the sugar unit of different acyl substituents such as:
- aliphatic acids, such as acetic, malic, succinic and malonic acid;
- cinnamic acids (aromatic substituents), such as sinapic, ferulic and p-coumaric acid;
- finally, there are pigments with both aromatic and aliphatic substituents.
Furthermore, some anthocyanins have several acylated sugars in the molecule; these anthocyanins are sometimes called polyglycosides.
Depending on the type of hydroxylation, methoxylation and glycosylation patterns, and the different substituents linked to the sugar units, more than 500 different anthocyanins have been identified that are based on 31 anthocyanidins. Among these 31 monomers:
- 30 percent are from cyanidin;
- 22 percent are from delphinidin;
- 18 percent are from pelargonidin.
Methylated derivatives of cyanidin, delphinidin and pelargonidin, namely peonidin, malvidin, and petunidin, all together represent 20 percent of the anthocyanins.
Therefore, up to 90 percent of the most frequently encountered anthocyanins are related to delphinidin, pelargonidin, cyanidin, and their methylated derivatives.
Role of pH
The color of these molecules is influenced by the pH of the vacuole where they are stored, ranging in color from:
- red, under very acidic conditions;
- to purple-blue, in intermediate pH conditions;
- until yellow-green, in alkaline conditions.
In addition to the pH, the color of these flavonoids can be affected by the degree of hydroxylation or methylation pattern of the A and B rings, and by glycosylation pattern.
Finally, the color of certain plant pigments result from complexes between anthocyanins, flavones and metal ions.
It should be noted that anthocyanins are often used as pH indicators thanks to the differences in chemical structure that occur in response to changes in pH.
References
- de la Rosa L.A., Alvarez-Parrilla E., Gonzàlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1th Edition. Wiley J. & Sons, Inc., Publication, 2010
- de Pascual-Teresa S., Moreno D.A. and García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 2010;11:1679-1703. doi:10.3390/ijms11041679
- Escribano-Bailòn M.T., Santos-Buelga C., Rivas-Gonzalo J.C. Anthocyanins in cereals. J Chromatogr A 2004:1054;129-141. doi:10.1016/j.chroma.2004.08.152
- Han X., Shen T. and Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci 2007;9:950-988. doi:10.3390/i8090950
- Manach C., Scalbert A., Morand C., Rémésy C., and Jime´nez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79(5):727-747. doi:10.1093/ajcn/79.5.727
- Ottaviani J.I., Kwik-Uribe C., Keen C.L., and Schroeter H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am J Clin Nutr 2012;95:851-858. doi:10.3945/ajcn.111.028340
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010;2:1231-1246. doi:10.3390/nu2121231