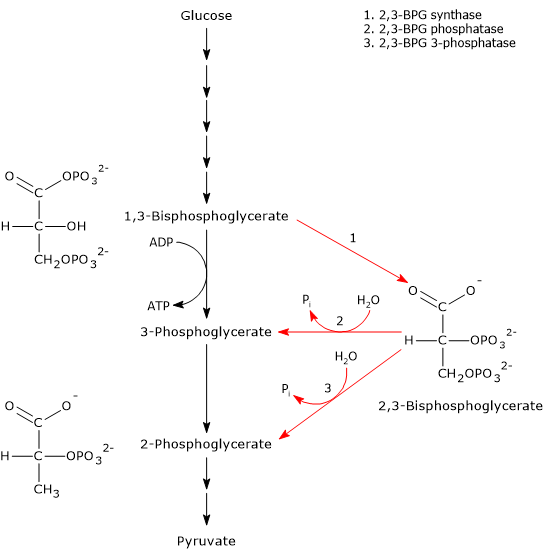
The Rapoport-Luebering shunt is a metabolic pathway predominantly present in red blood cells and is a side glycolytic pathway that branches off at the level of 1,3-bisphosphoglycerate (1,3-BPG).[1][2]
The two reactions of the shunt are catalyzed by a multifunctional enzyme, bisphosphoglycerate mutase (EC 5.4.2.4). However, a second enzyme involved in the shunt, 2,3-bisphosphoglycerate 3-phosphatase (EC 3.1.3.80), has recently been identified.[3]
The most important product is 2,3-bisphosphoglycerate (2,3-BPG), an allosteric effector of hemoglobin that reduces its affinity for molecular oxygen, thereby facilitating its release at the tissue level.[4][5]
As it branches off from glycolysis upstream of the reactions leading to ATP synthesis, the Rapoport-Luebering shunt imposes an energy cost on the cell.[3]
Defects in glycolytic enzymes in red blood cells lead to abnormalities in 2,3-BPG synthesis and, consequently, in the affinity of hemoglobin for molecular oxygen.[6]
Contents
- Bisphosphoglycerate mutase
- Reactions
- 2,3-Bisphosphoglycerate 3-phosphatase
- Role of the Rapoport-Luebering shunt
- Energy cost of the Rapoport-Luebering shunt
- Relationship between glycolysis and the Rapoport-Luebering shunt
- References
Bisphosphoglycerate mutase
Bisphosphoglycerate mutase is a central enzyme in the Rapoport-Luebering shunt, and is found in red blood cells.[4]
It is a multifunctional enzyme, as it functions as a 2,3-bisphosphoglycerate synthase and a 2,3-bisphosphoglycerate phosphatase. It is also a 2,3-bisphosphoglycerate mutase, an activity similar to that of glycolytic phosphoglycerate mutase (EC 5.4.2.1), but does not participate in the shunt. All three catalytic activities take place at the same active site, with synthase being the primary activity, which is inhibited by free 2,3-bisphosphoglycerate.[7][8]
 The phosphatase activity is low, approximately 1000 times lower than the synthase activity, and is stimulated by various anions, including chloride, phosphate, sulfite, and, more markedly, 2-phosphoglycolate.[9]
The phosphatase activity is low, approximately 1000 times lower than the synthase activity, and is stimulated by various anions, including chloride, phosphate, sulfite, and, more markedly, 2-phosphoglycolate.[9]
Finally, it appears that the mutase activity is physiologically insignificant, catalyzing about 5 percent of the reversible conversion of 3-phosphoglycerate to 2-phosphoglycerate.[10][11]
Bisphosphoglycerate mutase is one of the three isoforms of phosphoglycerate mutase found in mammals. The other two isoforms are phosphoglycerate mutase 1 or type M, present in muscle, and phosphoglycerate mutase 2 or type B, present in all other tissues. These two isoforms are also trifunctional enzymes.[4]
Reactions
The Rapoport-Luebering shunt involves two reactions.
In the first reaction, bisphosphoglycerate mutase acts as a synthase, catalyzing the isomerization of 1,3-BPG to 2,3-bisphosphoglycerate. The enzyme requires 3-phosphoglycerate, as it catalyzes the intermolecular transfer of a phosphate group from C-1 of 1,3-bisphosphoglycerate to C-2 of 3-phosphoglycerate. In the reaction, therefore, the starting 3-phosphoglycerate becomes 2,3-BPG, while 1,3-BPG becomes the new 3-phosphoglycerate.[12]
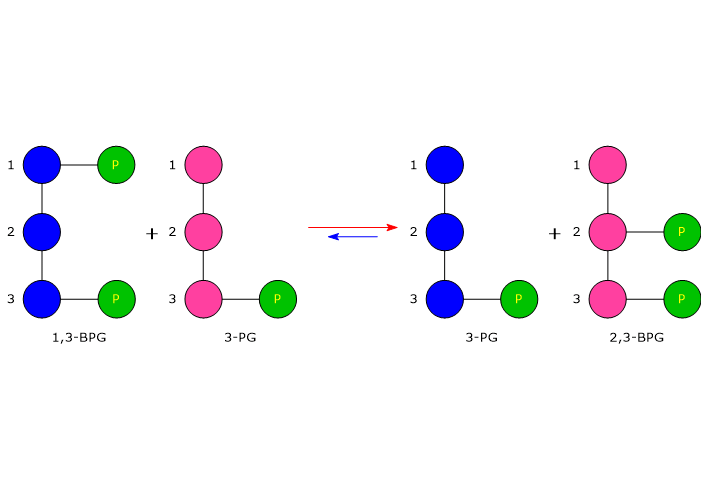 In the second step, the phosphatase activity of bisphosphoglycerate mutase catalyzes the synthesis of 3-phosphoglycerate, a glycolytic intermediate, following the hydrolysis of the phosphate group at position C-2 of 2,3-BPG. The 3-phosphoglycerate produced can then re-enter the glycolytic pathway at the reaction catalyzed by phosphoglycerate mutase, i.e., the eighth step of the pathway.[5]
In the second step, the phosphatase activity of bisphosphoglycerate mutase catalyzes the synthesis of 3-phosphoglycerate, a glycolytic intermediate, following the hydrolysis of the phosphate group at position C-2 of 2,3-BPG. The 3-phosphoglycerate produced can then re-enter the glycolytic pathway at the reaction catalyzed by phosphoglycerate mutase, i.e., the eighth step of the pathway.[5]
2,3-Bisphosphoglycerate 3-phosphatase
A study has identifying a second enzyme involved in the Rapoport-Luebering shunt. In addition to bisphosphoglycerate mutase, researchers have discovered a distinct 2,3-bisphosphoglycerate 3-phosphatase activity catalyzed by an evolutionarily conserved multiple inositol polyphosphate phosphatase, known as MIPP1, which has been found in Dictyostelium, birds, and mammals.
This discovery reveals that the formation of 3-phosphoglycerate, a precursor for serine biosynthesis and an activator of AMP-activated protein kinase, can be bypassed. By genetically manipulating MIPP1 expression in Dictyostelium, scientists demonstrated the enzyme’s physiologically relevant role in regulating cellular 2,3-BPG content.[3]
Role of the Rapoport-Luebering shunt
The Rapoport-Luebering shunt leads to the formation of 2,3-BPG, an allosteric effector of hemoglobin, along with carbon dioxide and hydrogen ions.[5]
2,3-Bisphosphoglycerate is found in high concentrations in human red blood cells, whereas in other cells, it is present only in trace amounts.[6] In most primates and many other mammals, it decreases hemoglobin’s affinity for molecular oxygen, thereby facilitating its release to the tissues.[3]
2,3-BPG binds to deoxyhemoglobin by means of ionic bonds with the β chains in the T state, a state in which these subunits form an electrostatic pocket complementary to both the conformation and the charge distribution of the molecule. The bond stabilizes the T state, hence deoxyhemoglobin, and is primarily responsible for hemoglobin’s cooperative binding of molecular oxygen.[12]
In trace amounts, 2,3-BPG is also required for the catalytic activity of phosphoglycerate mutase. It follows that another function of bisphosphoglycerate mutase, and hence of the Rapoport-Luebering shunt, is to control the level of glycolytic intermediates.[13]
Energy cost of the Rapoport-Luebering shunt
1,3-bisphosphoglycerate is an intermediate of the glycolytic pathway. In the seventh step of glycolysis, phosphoglycerate kinase (EC 2.7.2.3) catalyzes the transfer of the high-energy phosphoryl group at the C-1 position of 1,3-BPG to ADP, with the formation of ATP and 3-phosphoglycerate. The reaction, a substrate-level phosphorylation, is the first of two reactions in the glycolytic pathway in which part of the chemical energy contained in glucose is conserved in the form of ATP.[5]
Therefore, the entry of 1,3-BPG into the Rapoport-Luebering cycle incurs an energy cost equivalent to two ATP molecules per 1,3-bisphosphoglycerate molecule diverted from glycolysis.[3] As a result, a delicate balance exists between the energy demands of red blood cells and the requirement to maintain hemoglobin in an optimal state of oxygenation and deoxygenation.[14]
Relationship between glycolysis and the Rapoport-Luebering shunt
Under physiological conditions, the Rapoport-Luebering shunt intercepts approximately 20 percent of the glycolytic flux in red blood cells.[8] It follows that defects in erythrocyte glycolytic enzymes influence the shunt and, consequently, the affinity of hemoglobin for molecular oxygen.[6]
Two conditions that illustrate this connection are nonspherocytic hemolytic anemia due to hexokinase deficiency and hemolytic anemia due to pyruvate kinase deficiency in erythrocytes, both of which are autosomal recessive diseases.[15][16]
In nonspherocytic hemolytic anemia due to hexokinase deficiency there is a deficiency of erythrocyte hexokinase (EC 2.7.1.1).[17] The enzyme catalyzes the phosphorylation of glucose to glucose-6-phosphate. Its deficiency leads to a decrease in the erythrocyte concentration of 2,3-BPG and, consequently, an increase in the affinity of hemoglobin for molecular oxygen.[6]
In contrast, hemolytic anemia due to pyruvate kinase deficiency in erythrocytes is characterized by a deficiency of pyruvate kinase (EC 2.7.1.40), leading to increased synthesis of 2,3-BPG and a decreased affinity of hemoglobin for molecular oxygen.[18]
Reference
- ^ Rapoport S., Luebering J. Glycerate-2,3-diphosphatase. J Biol Chem 1951;189(2):683-694.
- ^ Rapoport S., Luebering J. The formation of 2,3-diphosphoglycerate in rabbit erythrocytes: the existence of a diphosphoglycerate mutase. J Biol Chem 1950;183(2):507-516.
- ^ a b c d e Cho J., King J.S., Qian X., Harwood A.J., Shears S.B. Dephosphorylation of 2,3-bisphosphoglycerate by MIPP expands the regulatory capacity of the Rapoport-Luebering glycolytic shunt. Proc Natl Acad Sci U S A 2008;105(16):5998-6003. doi:10.1073/pnas.0710980105
- ^ a b c DiMauro S., Miranda A.F., Khan S., Gitlin K., Friedman R. Human muscle phosphoglycerate mutase deficiency: newly discovered metabolic myopathy. Science 1981;212(4500):1277-9. doi:10.1126/science.6262916
- ^ a b c d Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012.
- ^ a b c d Voet D. and Voet J.D. Biochemistry. 4th Edition. John Wiley J. & Sons, Inc. 2011.
- ^ Aljahdali A.S., Musayev F.N., Burgner J.W. 2nd, Ghatge M.S., Shekar V., Zhang Y., Omar A.M., Safo M.K. Molecular insight into 2-phosphoglycolate activation of the phosphatase activity of bisphosphoglycerate mutase. Acta Crystallogr D Struct Biol 2022;78(Pt 4):472-482. doi:10.1107/S2059798322001802
- ^ a b Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012.
- ^ Reynolds C.H. Activation of human erythrocyte 2,3-bisphosphoglycerate phosphatase at physiological concentrations of substrate. Arch Biochem Biophys 1986;250(1):106-11. doi:10.1016/0003-9861(86)90706-x
- ^ Lemarchandel V., Joulin V., Valentin C., Rosa R., Galactéros F., Rosa J., Cohen-Solal M. Compound heterozygosity in a complete erythrocyte bisphosphoglycerate mutase deficiency. Blood 1992;80(10):2643-9. doi:10.1182/blood.V80.10.2643.2643
- ^ Sasaki R., Chiba H. Role and induction of 2,3-bisphosphoglycerate synthase. Mol Cell Biochem 1983;53-54(1-2):247-56. doi:10.1007/BF00225257
- ^ a b Garrett R.H., Grisham C.M. Biochemistry. 4th Edition. Brooks/Cole, Cengage Learning, 2010.
- ^ Oslund R.C., Su X., Haugbro M., Kee J-M., Esposito M., David Y., Wang B., Ge E., Perlman D.H., Kang Y., Muir T.W., Rabinowitz J.D. Bisphosphoglycerate mutase controls serine pathway flux via 3-phosphoglycerate. Nat Chem Biol 2017;13:1081-1087. doi:10.1038/nchembio.2453
- ^ Hodgkins S.R. 6 – Erythrocyte metabolism and membrane structure and function. Editor(s): Keohane E.M., Otto C.N., Walenga J.M. Rodak’s Hematology (Sixth Edition), Elsevier. 2020:78-90. doi:10.1016/B978-0-323-53045-3.00015-5
- ^ Orphanet: hemolytic anemia due to red cell pyruvate kinase deficiency. https://www.orpha.net/en/disease/detail/766
- ^ Orphanet: non-spherocytic hemolytic anemia due to hexokinase deficiency. https://www.orpha.net/en/disease/detail/90031
- ^ Paglia D.E., Shende A., Lanzkowsky P., Valentine W.N. Hexokinase “New Hyde Park”: a low activity erythrocyte isozyme in a Chinese kindred. Am J Hematol 1981;10(2):107-17. doi:10.1002/ajh.2830100202
- ^ Enegela O.A., Anjum F. Pyruvate Kinase Deficiency. [Updated 2023 Apr 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560581/