2,3-Bisphosphoglycerate (2,3-BPG) is the conjugated base of 2,3-bisphosphoglyceric acid.[1]
In red blood cells, at sea level, 2,3-BPG is found in concentrations close to that of hemoglobin (Hb), about 5 mM. In contrast, in other cell types, it is found only in trace amounts.[2]
In erythrocytes, it is synthesized from 1,3-bisphosphoglycerate (1,3-BPG) in the first reaction of the Rapoport-Luebering shunt.[3][4] Since the shunt is a detour from the glycolytic pathway, occurring upstream of the reactions leading to ATP synthesis, the production of 2,3-bisphosphoglycerate incurs an energy cost of one ATP molecule per molecule produced.[5]
2,3-BPG is one of the regulators of the binding affinity of hemoglobin for oxygen.[6] It is also required for the catalytic activity of phosphoglycerate mutase (EC 5.4.2.1), and is an allosteric activator of ribose phosphate pyrophosphokinase (EC 2.7.6.1).[2][7]
By binding to hemoglobin, it lowers its oxygen affinity, thereby facilitating oxygen release in peripheral tissues.[6]
In red blood cells, the concentration of 2,3-bisphosphoglycerate can vary in response to changes in the flow of carbon through the glycolytic pathway. These variations may result from changes in altitude, diseases that impair blood oxygenation, or defects in glycolytic enzymes, and they affect hemoglobin binding affinity for oxygen.[8][9][10]
Contents
- Chemical properties
- Metabolism
- Energy cost of 2,3-bisphosphoglycerate synthesis
- Role of 2,3-bisphosphoglycerate
- Hemoglobin
- 2,3-Bisphosphoglycerate and hemoglobin A
- 2,3-Bisphosphoglycerate and hemoglobin F
- Changes in 2,3-bisphosphoglycerate concentration
- References
Chemical properties
2,3-Bisphosphoglycerate is the conjugate base of 2,3-bisphosphoglyceric acid. It has a molecular weight of 261.00 g/mol, and molecular formula C3H3O10P2-5.[1]
According to the IUPAC nomenclature, its systematic name is (2R)-2,3-bis(phosphonooxy)propanoate.[11]
The pKa of 2,3-bisphosphoglyceric acid is 0.48. Therefore, at physiological pH, it exists almost exclusively in its ionized form, 2,3-BPG.[11]
It is soluble in water, with a solubility of 9.69 g/L.[1]
2,3-Bisphosphoglycerate is an isomer of 1,3-bisphosphoglycerate.
Metabolism
2,3-bisphosphoglycerate is produced from the glycolytic intermediate 1,3-bisphosphoglycerate in the first of the two steps of the Rapoport-Luebering shunt.[3][4]
Both reactions of the shunt are catalyzed by bisphosphoglycerate mutase (EC 5.4.2.4), a multifunctional enzyme with three main activities, 2,3-BPG synthase, its main activity, 2,3-BPG phosphatase, and 2,3-BPG mutase.[12]
In the first step of the Rapoport-Luebering shunt, bisphosphoglycerate mutase acts as a synthase and catalyzes the isomerization of 1,3-bisphosphoglycerate to 2,3-bisphosphoglycerate. The enzyme catalyzes the intermolecular transfer of a phosphate group from C-1 of 1,3-BPG to C-2 of 3-phosphoglycerate, which means that 3-phosphoglycerate must be present in the active site. During the reaction, 3-phosphoglycerate is converted into 2,3-bisphosphoglycerate, while 1,3-BPG is transformed into 3-phosphoglycerate.[6]
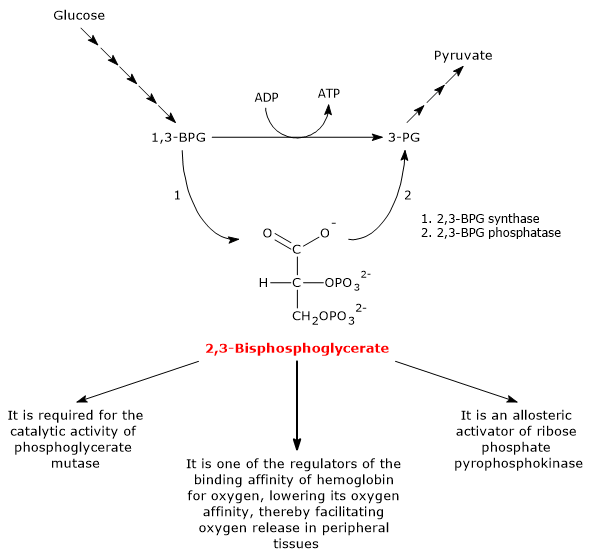 In the second step, 2,3-bisphosphoglycerate is dephosphorylated into 3-phosphoglycerate. The reaction is catalyzed by the phosphatase activity of bisphosphoglycerate mutase. 3-Phosphoglycerate then re-enters the glycolytic pathway at the eighth step, which is catalyzed by phosphoglycerate mutase (EC 5.4.2.1).[5]
In the second step, 2,3-bisphosphoglycerate is dephosphorylated into 3-phosphoglycerate. The reaction is catalyzed by the phosphatase activity of bisphosphoglycerate mutase. 3-Phosphoglycerate then re-enters the glycolytic pathway at the eighth step, which is catalyzed by phosphoglycerate mutase (EC 5.4.2.1).[5]
Energy cost of 2,3-bisphosphoglycerate synthesis
2,3-Bisphosphoglycerate synthesis incurs an energy cost for the red blood cell, equivalent to one ATP molecule per molecule produced.[5]
1,3-BPG is the substrate of the seventh reaction of glycolysis, in which phosphoglycerate kinase (EC 2.7.2.3) catalyzes the transfer of the high-energy phosphate group at the C-1 position to ADP, with the formation of 3-phosphoglycerate and ATP. Phosphoglycerate kinase catalyzes the first of two substrate-level phosphorylations that, along the glycolytic pathway, lead to the conservation of part of the chemical energy contained in glucose in the form of ATP, the cell’s energy currency.[6]
Since, under physiological conditions, the Rapoport-Luebering shunt intercepts approximately 20% of glycolytic carbon flux, the energy cost of 2,3-bisphosphoglycerate synthesis requires a finely tuned balance between the cell’s energy needs and the requirement to maintain hemoglobin in an optimal balance between deoxygenation and oxygenation.[13][14]
Role of 2,3-bisphosphoglycerate
2,3-bisphosphoglycerate has at least three biological functions.
- It is required for the catalytic activity of phosphoglycerate mutase. Consequently, the molecule also contributes to the regulation of the levels of glycolytic intermediates.[7]
- It is one of the allosteric activators of ribose phosphate pyrophosphokinase or PRPP synthase. The enzyme catalyzes the conversion of ribose-5-phosphate, one of the two products of the pentose phosphate pathway, into 5-phosphoribosyl-1-pyrophosphate (PRPP), an intermediate in the de novo synthesis of purines.[2]
- 2,3-BPG, along with other allosteric effectors, plays a role in the allosteric regulation of hemoglobin-oxygen affinity, facilitating oxygen release in peripheral tissues.[15] In humans, most primates, and many other mammals, 2,3-BPG reduces hemoglobin-oxygen affinity. In this way, 2,3-BPG promotes the dissociation of oxygen from hemoglobin, thus enhancing oxygen delivery to tissues.[6]
- The action of 2,3-BPG is an example of heterotropic allosteric regulation, i.e., allosteric regulation in which the effector is different from the normal ligand of the protein.[5]
Hemoglobin
Hemoglobin is found in red blood cells and, in almost all vertebrates, it is responsible for carrying oxygen to tissues.
The protein has a quaternary structure formed by four subunits and contains four heme prosthetic groups, one per subunit, each of which can reversibly bind an oxygen molecule.[13]
Under physiological conditions, two types of hemoglobin are found in humans: hemoglobin A and hemoglobin F, which is found in the fetus.
Hemoglobin A is composed of two α chains of 141 amino acid residues and two β chains of 146 amino acid residues. Therefore, it is an α2β2 tetramer.
In hemoglobin F, the β chains are replaced by γ chains, which are very similar, but not identical, to the β chains. The result is an α2γ2 stoichiometry.[13] This difference has a major influence on the protein’s affinity for oxygen.[5]
Hemoglobin can exist in two conformations known as the T (tense) and R (relaxed) states.
Although oxygen binds to hemoglobin in both the T and R states, it has a greater affinity for the R state, which is stabilized by the binding. The binding of oxygen by hemoglobin is modulated by several factors, some exerting short-term effects and others, such as 2,3-bisphosphoglycerate, exerting long-term effects.[6]
Cooperativity of hemoglobin-oxygen binding
Hemoglobin in the deoxygenated state is in the T conformation. Oxygen binding to hemoglobin in the T state can occur only on the heme group of one α subunit, since the heme groups of the β subunits in the T state are virtually inaccessible.[6]
The binding of the first oxygen molecule triggers a conformational change in the α subunit, causing its conversion to the R state. This change is transmitted to adjacent subunits via subunit-subunit interactions, which then cause the second α subunit to switch to the R state. This triggers sequential conformational changes that lead to the transition of hemoglobin’s quaternary structure, which binds two oxygen molecules, Hb(O2)2, from the T state to the R state. At this point, the β subunits are now able to bind oxygen.[6]
This type of binding is called cooperative binding and is the basis of the sigmoidal shape of the hemoglobin-oxygen binding curve.[16]
Hemoglobin affinity for oxygen
In the lung capillaries, oxygen binds to hemoglobin and through the bloodstream reaches the peripheral tissues, where it is released.
The affinity between hemoglobin and oxygen is mainly determined by the structure of hemoglobin. However, it is modulated by various factors such as:
- temperature;
- pH;
- carbon dioxide (CO2);
- chloride ions (Cl⁻);
- 2,3-bisphosphoglycerate.[15]
Changes in the affinity of the Hb-O2 bond that occur in the circulatory system due to the above factors optimize oxygen uptake in the lungs and its release in peripheral tissues.[10]
Graphically, changes in binding affinity induced by allosteric effectors and temperature translate into shifts of the hemoglobin-oxygen binding curve to the right, in the case of a decrease in binding affinity, or to the left, when binding affinity increases.[5]
Temperature, pH, and CO2 exert short-term effects.[10] For example, in skeletal muscle during physical activity, temperature, the concentration of hydrogen ions (H⁺) and partial pressure of CO2 increase. High concentrations of H⁺ and CO2 lower hemoglobin affinity for oxygen, an effect known as the Bohr effect. An increase in temperature also decreases affinity. These factors collectively favor the release of oxygen to the muscle tissue.[17]
Cl⁻ and 2,3-bisphosphoglycerate also decrease hemoglobin affinity for oxygen, but they are involved in long-term modulation.[10]
2,3-Bisphosphoglycerate and hemoglobin A
2,3-Bisphosphoglycerate binds to hemoglobin A through ionic bonds with positively charged residues of the β chains.
The bonds between 2,3-bisphosphoglycerate and hemoglobin involve the side chains of lysine 82, histidine 143, and the N-terminal amino group of each of the two β chains. These residues form an electrostatic pocket that is complementary to both the conformation and the charge distribution of 2,3-BPG.[5]
2,3-BPG binds to deoxygenated hemoglobin and stabilizes the T state. H⁺, Cl–, and CO2 also stabilize the T state.[10]
During the transition from the T to the R state, among the conformational changes occurring in the quaternary structure of hemoglobin, the binding pocket for 2,3-BPG between the β subunits narrows, preventing its binding. Notably, while hemoglobin has four binding sites for molecular oxygen, it has only one for 2,3-BPG.[6]
Furthermore, the binding site for 2,3-BPG is located far from that of oxygen.[5]
2,3-Bisphosphoglycerate and hemoglobin F
The γ chains of hemoglobin F, like the β chains of hemoglobin A, form the binding pocket for 2,3-BPG.[5]
However, there are two fewer positively charged residues in the binding pocket than in hemoglobin A. In fact, at position 143 of the γ chains, a histidine is replaced by a serine.[2]
This small difference in the primary structure means that 2,3-bisphosphoglycerate binds with lower affinity, and consequently, hemoglobin F has a higher oxygen affinity than maternal hemoglobin. This helps ensure the transfer of oxygen from hemoglobin A to hemoglobin F, and thus from the maternal circulation to the fetal circulation.[6]
Changes in 2,3-bisphosphoglycerate concentration
The concentration of 2,3-bisphosphoglycerate in red blood cells can be influenced by various factors, both physiological and pathological.[2]
Physiological conditions include the adaptation to high altitude, while pathological conditions include genetic defects affecting glycolytic enzymes, both downstream and upstream of the reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12), and diseases that cause hypoxia.[18][19]
In all cases, changes are due to variations in the rate of 2,3-BPG synthesis and are reflected in hemoglobin-oxygen affinity.
Adaptation to high altitudes
Adaptation to high altitude is a rather complex process that also involves an increase in the number of red blood cells and their hemoglobin content. These changes require weeks to complete. However, after just one day at high altitude, some degree of adaptation is perceived. This effect is a consequence of the increase in 2,3-bisphosphoglycerate concentration in red blood cells.[2]
What happens is that the low partial pressure of oxygen leads to an increase in blood pH (alkalosis) which stimulates glycolysis. This, in turn, leads to an increased carbon flow through the Rapoport-Luebering shunt. This causes an increase in the synthesis of 2,3-bisphosphoglycerate, whose concentration can reach up to 8 mM. Such increase leads to a decrease in hemoglobin-oxygen affinity, facilitating oxygen release to the peripheral tissues and thus contributing to adaptation to high altitudes.[5]
Anemia
Variations in erythrocyte 2,3-bisphosphoglycerate concentration also occur in two autosomal recessive diseases: non-spherocytic hemolytic anemia due to hexokinase deficiency and hemolytic anemia due to red cell pyruvate kinase deficiency.[18][19]
In hemolytic anemia due to red cell pyruvate kinase (EC 2.7.1.40) deficiency, enzyme deficiency causes an increase in the synthesis of 2,3-BPG and, consequently, a reduction in hemoglobin-oxygen affinity.[8] An increase in 2,3-BPG concentration also occurs in individuals affected by conditions that limit blood oxygenation, such as hypoxia-inducing conditions. An example is cardiopulmonary failure.[2]
In non-spherocytic hemolytic anemia due to hexokinase deficiency (EC 2.7.1.1), the enzyme deficiency reduces 2,3-bisphosphoglycerate concentration in red blood cells, leading to an increase in hemoglobin-oxygen affinity.[9]
References
- ^ a b c National Center for Biotechnology Information. PubChem Compound Summary for CID 9548671, 2,3-Bisphosphoglycerate. https://pubchem.ncbi.nlm.nih.gov/compound/2_3-Bisphosphoglycerate. Accessed Feb. 16, 2025.
- ^ a b c d e f g Heilman D., Woski S., Voet D., Voet J.G., Pratt C.W. Fundamentals of biochemistry: life at the molecular level. 6th Edition. Wiley, 2023.
- ^ a b Rapoport S., Luebering J. Glycerate-2,3-diphosphatase. J Biol Chem 1951;189(2):683-694.
- ^ a b Rapoport S., Luebering J. The formation of 2,3-diphosphoglycerate in rabbit erythrocytes: the existence of a diphosphoglycerate mutase. J Biol Chem 1950;183(2):507-516.
- ^ a b c d e f g h i j Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 8th Edition. W.H. Freeman and Company, 2021.
- ^ a b c d e f g h i j Garrett R.H., Grisham C.M. Biochemistry. 6th Edition. Brooks/Cole, Cengage Learning, 2016.
- ^ a b Oslund R.C., Su X., Haugbro M., Kee J-M., Esposito M., David Y., Wang B., Ge E., Perlman D.H., Kang Y., Muir T.W., Rabinowitz J.D. Bisphosphoglycerate mutase controls serine pathway flux via 3-phosphoglycerate. Nat Chem Biol 2017;13:1081-1087. doi:10.1038/nchembio.2453
- ^ a b Enegela O.A., Anjum F. Pyruvate Kinase Deficiency. [Updated 2023 Apr 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560581/
- ^ a b Paglia D.E., Shende A., Lanzkowsky P., Valentine W.N. Hexokinase “New Hyde Park”: a low activity erythrocyte isozyme in a Chinese kindred. Am J Hematol 1981;10(2):107-17. doi:10.1002/ajh.2830100202
- ^ a b c d e Webb K.L., Dominelli P.B., Baker S.E., Klassen S.A., Joyner M.J., Senefeld J.W., Wiggins C.C. Influence of high hemoglobin-oxygen affinity on humans during hypoxia. Front Physiol 2022;12:763933. doi:10.3389/fphys.2021.763933
- ^ a b HMDB. Metabocard for 2,3-Diphosphoglyceric acid. https://hmdb.ca/metabolites/HMDB0001294
- ^ Aljahdali A.S., Musayev F.N., Burgner J.W. 2nd, Ghatge M.S., Shekar V., Zhang Y., Omar A.M., Safo M.K. Molecular insight into 2-phosphoglycolate activation of the phosphatase activity of bisphosphoglycerate mutase. Acta Crystallogr D Struct Biol 2022;78(Pt 4):472-482. doi:10.1107/S2059798322001802
- ^ a b c Moran L.A., Horton H.R., Scrimgeour K.G., Perry M.D. Principles of Biochemistry. 5th Edition. Pearson, 2012.
- ^ Hodgkins S.R. 6 – Erythrocyte metabolism and membrane structure and function. Editor(s): Keohane E.M., Otto C.N., Walenga J.M. Rodak’s Hematology (Sixth Edition), Elsevier. 2020:78-90. doi:10.1016/B978-0-323-53045-3.00015-5
- ^ a b Mairbäurl H., Oelz O., Bärtsch P. Interactions between Hb, Mg, DPG, ATP, and Cl determine the change in Hb-O2 affinity at high altitude. J Appl Physiol 1993;74(1):40-8. doi:10.1152/jappl.1993.74.1.40
- ^ Henry E.R., Bettati S., Hofrichter J., Eaton W.A. A tertiary two-state allosteric model for hemoglobin. Biophys Chem 2002;98(1-2):149-64. doi:10.1016/s0301-4622(02)00091-1
- ^ Böning D., Schwiegart U., Tibes U., Hemmer B. Influences of exercise and endurance training on the oxygen dissociation curve of blood under in vivo and in vitro conditions. Eur J Appl Physiol Occup Physiol 1975;34(1):1-10. doi:10.1007/BF00999910
- ^ a b Orphanet: hemolytic anemia due to red cell pyruvate kinase deficiency. https://www.orpha.net/en/disease/detail/766
- ^ a b Orphanet: non-spherocytic hemolytic anemia due to hexokinase deficiency. https://www.orpha.net/en/disease/detail/90031