Acidity regulators or pH control agents are additives used in processed foods to modify or stabilize their acidity or alkalinity level, which is measured by their pH value. Moreover, their action often overlaps with that of other food additives, as some can act as preservatives, others as emulsifiers, and some help maintain the color of the food to which they are added. Therefore, they contribute, along with other factors, to maintaining or improving the organoleptic properties of the product.[3]
Like other food additives, acidity regulators are identified by the E-numbering system, where E stands for Europe, in addition to their chemical name.[4]
Based on the scientific evidence currently available, when used according to specific guidelines, acidity regulators are not harmful to human health.[2]
Contents
What are they used for?
Acidity regulators are used in a wide range of foods. Their main functions are listed below.[3]
- pH Stabilization.
Some foods require an acidic or neutral environment to maintain their chemical integrity and, thus, their organoleptic properties. For example, carbonated drinks need acids to prevent changes in taste and composition during storage. - Food preservation.
Acidity helps prevent the growth of harmful microorganisms such as bacteria and mold. This is particularly important in packaged foods and preserves, where a low pH acts as a natural barrier against bacterial growth. In this sense, acidity regulators act as preservatives. - Flavor enhancement.
Acidity regulators can balance the flavors of foods by adding a sour note that enhances the overall taste. For example, lactic acid (E270) is used in dairy products and fermented foods to improve flavor. - Support for consistency and quality.
By regulating acidity, this class of food additives can help stabilize the structure of certain foods, such as cheeses, sweets, and ice cream, acting in a similar way to emulsifiers. - Enhancement of antioxidant action.
Some acidity regulators, like citric acid (E330), though not antioxidants in themselves, can enhance the activity of many antioxidants.
Examples of acidity regulators
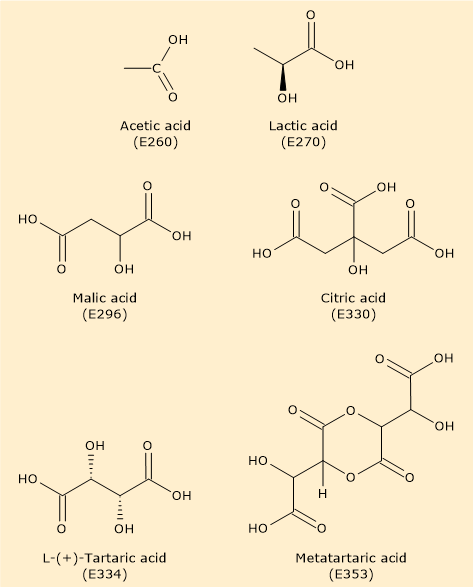
Below is an overview of some of the acidity regulators included in the list of food additives authorized for use in the European Union under Regulation 1129/2011 of the European Commission, published on November 11, 2011. This list is an amendment to Annex II of Regulation (EC) No. 1333/2008 of the European Parliament and was itself revised in 2013.[1]
| E-number | Additive and derivatives | |
| Acetic acid and its salts | ||
| E260 | Acetic acid | |
| E261 | Potassium acetate | |
| E262 | Sodium acetate | |
| E263 | Calcium acetate | |
| Lactic acid and its salts | ||
| E270 | Lactic acid | |
| E325 | Sodium lactate | |
| E326 | Potassium lactate | |
| E327 | Calcium lactate | |
| Citric acid and its salts | ||
| E330 | Citric acid | |
| E331 | Sodium citrate | |
| E332 | Potassium citrate | |
| E333 | Calcium citrate | |
| E380 | Triammonium citrate | |
| Tartaric acid and its salts | ||
| E334 | L-(+)-Tartaric acid | |
| E335 | Sodium tartrate | |
| E336 | Potassium tartrate | |
| E337 | Sodium potassium tartrate | |
| E353 | Metatartaric acid | |
| E354 | Calcium tartrate | |
| Malic acid and its salts | ||
| E296 | Malic acid | |
| E350 | Sodium malate | |
| E351 | Potassium malate | |
| E352 | Calcium malate | |
Health effects
As with other food additives, the safety of acidity regulators is assessed by the competent authorities, whose evaluations are based on the scientific literature available at the time of the assessment.
 Based on scientific evidence, when used according to specific guidelines, no health risks have been identified. However, like all additives, acidity regulators are subject to periodic safety reassessment.[2]
Based on scientific evidence, when used according to specific guidelines, no health risks have been identified. However, like all additives, acidity regulators are subject to periodic safety reassessment.[2]
References
- ^ Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. https://eur-lex.europa.eu/eli/reg/2011/1129/2013-11-21
- ^ a b EFSA Food additives: EFSA’s new guidance for applicants. Published: 18 July 2012. https://www.efsa.europa.eu/en/press/news/120718a
- ^ a b EUFIC. What are acidity regulators and why are they added to food. Last Updated: 01 December 2021. https://www.eufic.org/en/whats-in-food/article/acidity-regulators-the-multi-task-players
- ^ Food Standards Agency. Approved additives and E Numbers. Last updated: 23 January 2024.