Preservatives are additives used in foods that are at high risk of deterioration caused by microorganisms such as yeasts, bacteria, and moulds. Their action extends the shelf life of food products, ensures their safety, and helps prevent quality loss.[1][2]
There are different types of preservatives, each with a specific mode of action.[3]
Like other food additives, preservatives are identified by their name and/or by a code number in the E-numbering system, where “E” stands for Europe. They are listed from E200 to E299.[4]
Based on the scientific evidence currently available, food additives are not considered harmful to human health. However, particular attention should be paid to nitrates and nitrites. These molecules are not inherently dangerous, but can become so after chemical modifications that lead to the formation of N-nitrosamines, or simply nitrosamines, compounds regarded as carcinogenic to humans.[5][6]
Contents
- How preservatives work
- Foods they are added to
- Examples of preservatives
- Health effects of nitrates and nitrites
- References
How preservatives work
Preservatives act in several ways, but all are aimed at inhibiting the growth of pathogenic microorganisms.[7]
Their mechanisms of action include disrupting cell membranes, inhibiting enzymes, preventing bacterial spore germination, and forming inhibitory compounds.[8]
Food spoilage can also be caused by non-microbial factors, such as chemical agents like oxidants (primarily oxygen), and physical agents, such as light and temperature.[9]
To prevent this type of spoilage, antioxidants are added to food. From this perspective, they can also be considered preservatives. However, in this case, the role of the additive is to prevent changes in flavor and/or appearance, not to act as an antimicrobial, a role performed instead by preservatives.[3]
On the other hand, certain preservatives, such as nitrates and sulphites, also act as antioxidants and, in some cases, as food colours.[3] For example, nitrates and nitrites help maintain the red color of meat and contribute to the development of its “cured” flavor.[10]
Foods they are added to
Different types of preservatives are used mainly in highly perishable foods, such as fishery products (fish, mollusks, and crustaceans), as well as in meat and dairy products.
Organic acids, such as propionic acid (E280), benzoic acid (E210), sorbic acid (E200), and their salts, are added to foods with a low pH. For example, sorbic acid and its potassium and calcium salts inhibit the growth of molds, yeasts, and fungi in products such as wine, fruit juices, processed cheeses, and some baked goods like pastries.
The antimicrobial activity of sorbic acid increases as the pH of the medium decreases, since the fully protonated molecule is readily absorbed by microorganisms.[11]
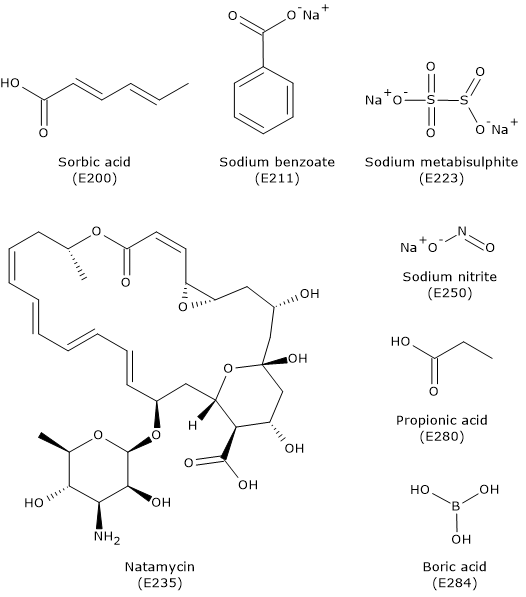
Nitrates and nitrites, specifically their sodium and potassium salts (E249–E252), are added to processed meats such as sausages, sliced meats, canned meats, and certain cheeses. They are particularly effective at inhibiting the germination of Clostridium botulinum spores, the bacterium that causes botulism.[10]
Sulphur dioxide and sulphites (E220-E228) are added to products such as wines, dried fruits, and fruit juices.[7][12]
Nisin (E234), a polycyclic polypeptide produced by the bacterium Lactococcus lactis and consisting of 34 amino acids, is a broad-spectrum bacteriocin effective against many Gram-positive bacteria and spores.[13] It is added to foods such as beverages, processed cheeses, and processed meats.[14]
Natamycin, also known as NATA (E235), is a preservative that acts on molds and yeasts. It is used, for example, for the surface treatment of dairy products and processed meats such as sausages. It is produced by certain bacterial species belonging to the genus Streptomyces.[15][16][17]
Examples of preservatives
Below is a brief overview of some preservatives included in the list of food additives approved by the European Community, according to Regulation 1129/2011 of the European Commission, published on November 11, 2011. This list amends Annex II to Regulation (EC) No. 1333/2008 of the European Parliament, and it was further updated in 2013.[1]
| E-number | Additive and derivatives |
|---|---|
| Sorbic acid and its salts | |
| E200 | Sorbic acid |
| E202 | Potassium sorbate |
| E203 | Calcium sorbate |
| Benzoic acid and its salts | |
| E210 | Benzoic acid |
| E211 | Sodium benzoate |
| E212 | Potassium benzoate |
| E213 | Calcium benzoate |
| E214 | Ethyl p-hydroxybenzoate |
| E215 | Sodium ethyl p-hydroxybenzoate |
| E218 | Methyl p-hydroxybenzoate |
| E219 | Sodium methyl p-hydroxybenzoate |
| Sulphur dioxide and its salts | |
| E220 | Sulphur dioxide |
| E221 | Sodium sulphite |
| E222 | Sodium hydrogen sulphite |
| E223 | Sodium metabisulphite |
| E224 | Potassium metabisulphite |
| E226 | Calcium sulphite |
| E227 | Calcium hydrogen sulphite |
| E228 | Potassium hydrogen sulphite |
| Other preservatives | |
| E234 | Nisin |
| E235 | Natamycin |
| E239 | Hexamethylenetetramine (hexamine) |
| E242 | Dimethyl dicarbonate |
| Nitrates and nitrites | |
| E249 | Potassium nitrite |
| E250 | Sodium nitrite |
| E251 | Sodium nitrate |
| E252 | Potassium nitrate (saltpeter) |
| Acids and their salts | |
| E280 | Propionic acid |
| E281 | Sodium propionate |
| E282 | Calcium propionate |
| E283 | Potassium propionate |
| E284 | Boric acid |
| E285 | Sodium tetraborate (borax) |
Health effects of nitrates and nitrites
In humans, the main sources of nitrates are water and certain vegetables, such as chard, arugula, lettuce, turnips, cabbage, celery, radishes, and spinach. The main source of exogenous nitrites, instead, is processed meat.[18]
Nitrates and nitrites are rapidly absorbed in the intestine and mostly excreted as nitrites. However, approximately 25% of absorbed nitrates enter the salivary glands, and about 5% is then released into the oral cavity, where bacterial reductases reduce them to nitrites.[19]
Once in the stomach, due to the acidic environment, nitrites can be converted into nitrous acid, which readily reacts with amines, molecules present in protein-rich foods such as meat products and cheese, to form nitrosamines, carcinogenic compounds. For these reasons, the International Agency for Research on Cancer has classified nitrates and nitrites as “probably carcinogenic to humans” (Group 2A).[20][21]
Therefore, excessive and prolonged consumption of processed foods containing nitrates and nitrites should be avoided, or at least strictly limited, as it has also been associated with an increased risk of esophageal and stomach cancer. However, the use of nitrates and nitrites is justified by the fact that, in small regulated amounts, their benefits in preventing contamination by Clostridium botulinum outweigh the potential cancer risk.[22]
It is important to note that the intake of antioxidants, such as vitamin C (E300) and its sodium and calcium salts (E301 and E302), inhibits the formation of N-nitrosamines from nitrates and nitrites.[23]
References
- ^ a b Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. https://eur-lex.europa.eu/eli/reg/2011/1129/2013-11-21
- ^ European Commission. Food safety. Additives. https://food.ec.europa.eu/food-safety/food-improvement-agents/additives_en
- ^ a b c García-García R., Searle S.S. Preservatives: food use. Encyclopedia of Food and Health. 2016:505-509. doi:10.1016/B978-0-12-384947-2.00568-7
- ^ Food Standards Agency. Approved additives and E Numbers. Last updated: 16 July 2025.
- ^ Jakszyn P., Gonzalez C.A. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol 2006;12(27):4296-303. doi:10.3748/wjg.v12.i27.4296
- ^ Ghasemi M., Bahrami Koutenaei M., Ghasemi A., Alizadeh-Navaei R., Moosazadeh M. A systematic review and dose-response meta-analysis of the association between nitrate & nitrite intake and gastroesophageal cancer risk. Nitric Oxide 2024;153:61-71. doi:10.1016/j.niox.2024.10.007
- ^ a b Singh R.P., Davidson P.M. Food additive. Encyclopedia Britannica, 24 Jun. 2025. https://www.britannica.com/topic/food-additive. Accessed 18 August 2025.
- ^ Davidson P.M., Taylor T.M., Schmidt S.E. Chemical preservatives and natural antimicrobial compounds. Food Microbiology: Fundamentals and Frontiers. 3rd ed. ASM Press, 2012. doi:10.1128/9781555818463.ch30
- ^ EUFIC. What are preservatives and what are common examples used in food? Last Updated: 01 October 2022.
- ^ a b Food Standards Agency. Food additives. Last updated: 16 July 2025. https://www.food.gov.uk/safety-hygiene/food-additives
- ^ Zeece M. Chapter Seven. Food additives. Introduction to the chemistry of food. Academic Press. 2020:251-311. doi:10.1016/B978-0-12-809434-1.00007-4
- ^ EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation sulfur dioxide (E220), sodium sulfite (E221), sodium bisulfite (E222), sodium metabisulfite (E223), potassium metabisulfite (E224), calcium sulfite (E226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives. EFSA Journal 2016;14(4):4438 151 pp. doi:10.2903/j.efsa.2016.4438
- ^ And H.C., Hoover D.G. Bacteriocins and their food applications. Compr Rev Food Sci Food Saf 2003;2(3):82-100. doi:10.1111/j.1541-4337.2003.tb00016.x
- ^ Gharsallaoui A., Oulahal N., Joly C., Degraeve P. Nisin as a food preservative: Part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr 2016;56(8):1262-74. doi:10.1080/10408398.2013.763765
- ^ Branen A.L., Davidson P.M., Salminen S., Thorngate J. Food Additives. 2nd Edition. CRC Press. 2001.
- ^ EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the use of natamycin (E235) as a food additive. EFSA Journal 2009;7(12):1412. doi:10.2903/j.efsa.2009.1412
- ^ Meena M., Prajapati P., Ravichandran C., Sehrawat R. Natamycin: a natural preservative for food applications-a review. Food Sci Biotechnol 2021;30(12):1481-1496. doi:10.1007/s10068-021-00981-1
- ^ Hord N.G., Tang Y., Bryan N.S. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 2009;90(1):1-10. doi:10.3945/ajcn.2008.27131
- ^ EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS). Re-evaluation of sodium nitrate (E251) and potassium nitrate (E252) as food additives. EFSA J 2017;15(6):e04787. doi:10.2903/j.efsa.2017.4787
- ^ Grosse Y., Baan R., Straif K., Secretan B., El Ghissassi F., Cogliano V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol 2006;7(8):628-9. doi:10.1016/s1470-2045(06)70789-6
- ^ Habermeyer M., Roth A., Guth S., Diel P., Engel K.H., Epe B., Fürst P., Heinz V., Humpf H.U., Joost H.G., Knorr D., de Kok T., Kulling S., Lampen A., Marko D., Rechkemmer G., Rietjens I., Stadler R.H., Vieths S., Vogel R., Steinberg P., Eisenbrand G. Nitrate and nitrite in the diet: how to assess their benefit and risk for human health. Mol Nutr Food Res 2015;59(1):106-28. doi:10.1002/mnfr.201400286
- ^ AIRC. Gli additivi e i conservanti alimentari aumentano il rischio di tumori? Ultimo aggiornamento: 22 marzo 2023. https://www.airc.it/cancro/informazioni-tumori/corretta-informazione/additivi-conservanti-alimentari
- ^ Tannenbaum S.R., Wishnok J.S., Leaf C.D. Inhibition of nitrosamine formation by ascorbic acid. Am J Clin Nutr 1991;53(1 Suppl):247S-250S. doi:10.1093/ajcn/53.1.247S