Glycogen synthesis, aka glycogenesis, is the constructive phase of glycogen metabolism.
It occurs through a different metabolic pathway than its breakdown, glycogenolysis, and restores glycogen reserves in the liver and skeletal muscle when carbohydrates are available.
In the liver it occurs in the fed state and it is stimulated both by increased glucose availability and insulin.
In post-exercise skeletal muscle, glycogen synthesis is faster when there are high blood glucose levels and insulin is available. Insulin stimulates glucose transport into muscle cells, thanks to the mobilization of specific transporters called GLT4, and glycogen synthase (EC 2.4.1.11) activity.
Glycogen synthesis requires more energy than that recovered during its breakdown: two ATP molecules are spent versus only one ATP molecules saved thanks to glucose-1-phoshate production. Therefore, the energy cost that the cell pays to store glucose as glycogen is an high-energy phosphate bond for each glucose unit.
Contents
Steps in glycogen synthesis
The first step in glycogen synthesis is glucose activation to glucose-6-phosphate, in the reaction catalyzed by glucokinase (EC 2.7.1.2) in the liver and hexokinase (EC 2.7.1.1) in the muscle and the other organs and tissues.
glucose + ATP → glucose-6-phosphate + ADP
When glycogen synthesis is occurring phosphoglucomutase (EC 5.4.2.2), the same enzyme that acts also during glycogenolysis catalyzing the conversion of glucose 1-phosphate to glucose-6-phosphate, shifts the phosphate group from C6 to C1 (therefore the enzyme catalyses a reversible reaction).
glucose-6-phosphate → glucose-1-phosphate
The next step is UDP-glucose production from glucose-1-phosphate and UTP in the reaction catalyzed by UDP-glucose pyrophosphorylase (EC 2.7.7.9); the reaction, reversible (and the name of the enzyme is due to the reverse reaction), becomes irreversible thanks to the rapid hydrolysis of pyrophosphate to inorganic phosphate in the reaction catalyzed by pyrophosphatase (EC 3.6.1.1).
glucose-1-phosphate + UTP → UDP-glucose + PPi
PPi + H2O → 2 Pi
It should be noted that thus far 2 ATP molecules are consumed per molecule of glucose activated to UDP-glucose: one for glucose-1-phosphate production and the other for the re-synthesis of UPT from UDP in the reaction catalyzed by nucleoside diphosphate kinase (EC 2.7.4.6).
UDP + ATP → UTP + ADP
Glycogen synthase transfers the activated glucose to 4’-OH group of a glucose residue (a nonreducing termini) present in the molecule catalyzing the formation of an α-(1,4) glycosidic bond and therefore extending the chain by one glucose unit. Therefore, like starch synthase and starch phosphorylase (EC 2.4.1.1), enzymes involved in the synthesis of amylose and amylopectin, the two polysaccharides that make up starch, glycogen synthase is a glycosyltransferase (EC 2.4).
The overall balanced equation for glycogen synthesis is:
glycogen(n glucose residues) + glucose + 2 ATP → glycogen(n+1 glucose residues) + 2 ADP + 2 Pi
The branches are inserted in the reaction catalyzed by the branching enzyme, also called amylo-α-(1,4)→α-(1,6)-transglucosidase (EC 2.4.1.18), that catalyzes the transfer en bloc of an oligosaccharide of six to seven glucose units from a nonreducing termini of a newly elongated chain of at least eleven units to another chain forming a new α-(1,6) glycosidic bond.
The new branches are introduced at least at four glucose residues from an adjacent branch point.
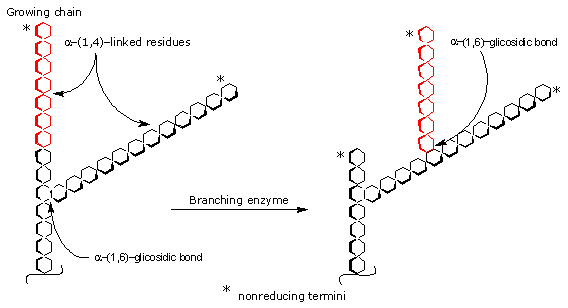
Then, glycogen synthase may add further glucose residues to the new branch.
References
- Adeva-Andany M.M., González-Lucán M., Donapetry-García C., Fernández-Fernández C., Ameneiros-Rodríguez E. Glycogen metabolism in humans. BBA Clin 2016;5:85-100. doi:10.1016/j.bbacli.2016.02.001
- Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012
- Stipanuk M.H., Caudill M.A. Biochemical, physiological, and molecular aspects of human nutrition. 3rd Edition. Elsevier health sciences, 2012