alpha-Linolenic acid or alpha-linolenic acid (18 carbon atoms), from the Latin linon, meaning flax, plus oleic, meaning oil or olive oil, was isolated by Hazura K. and Monatsh in 1887.
The exact structure was clarified by Erdmann E. et al in 1909 and was synthesized by Nigama and Weedon in 1956.
It is a polyunsaturated fatty acid (PUFA) with three cis double bounds (the first one from the methyl end is in omega-3 (ω-3) or n-3, so in shorthand notation is 18:3n-3) member of the sub-group called long chain fatty acids (LCFAs), from 14 to 18 carbon atoms.
PROPERTIES
Molecular weight: 278.4296 g/mol
Molecular formula: C18H30O2
IUPAC name: (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid
CAS registry number: 463-40-1
PubChem: 5280934
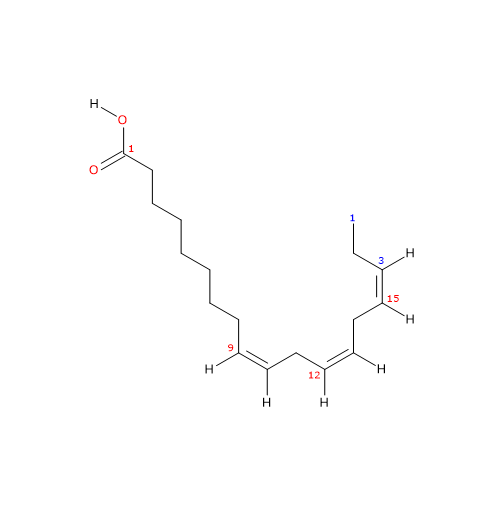
In purified form it is a colorless liquid insoluble in water, with melting point from -11.3 °C (11.66 °F; 261.85 K) to -11 °C (12.2 °F; 262.15 K) and boiling point at 230 (446 °F; 503.15 K)-232 °C (449.6 °F; 505.15 K) at 1 mm Hg.
OTHER NAMES
ALA
9Z,12Z,15Z-octadecatrienoic acid
all-cis-9,12,15-octadecatrienoic acid
cis,cis,cis-9,12,15-octadecatrienoic acid
9,12,15-octadecatrienoic acid, (Z,Z,Z)-
(Z,Z,Z)-9,12,15-octadecatrienoic acid
9-cis,12-cis,15-cis-octadecatrienoic acid
9,12,15-all-cis-octadecatrienoic acid
cis-delta 9,12,15-octadecatrienoic acid
(all-Z)-9,12,15-octadecatrienoic acid
(Z,Z,Z)-octadeca-9,12,15-trienoic acid
cis-9,cis-12,cis-15-octadecatrienoic acid
(9Z,12Z,15Z)-9,12,15-octadecatrienoic acid
18:3n-3
Metabolism of alpha-linolenic acid
It is produced de novo from linoleic acid, in a desaturation reaction catalyzed by Δ15-desaturase.
In turn linoleic acid is formed from oleic acid in a desaturation reaction catalyzed by Δ12-desaturase.
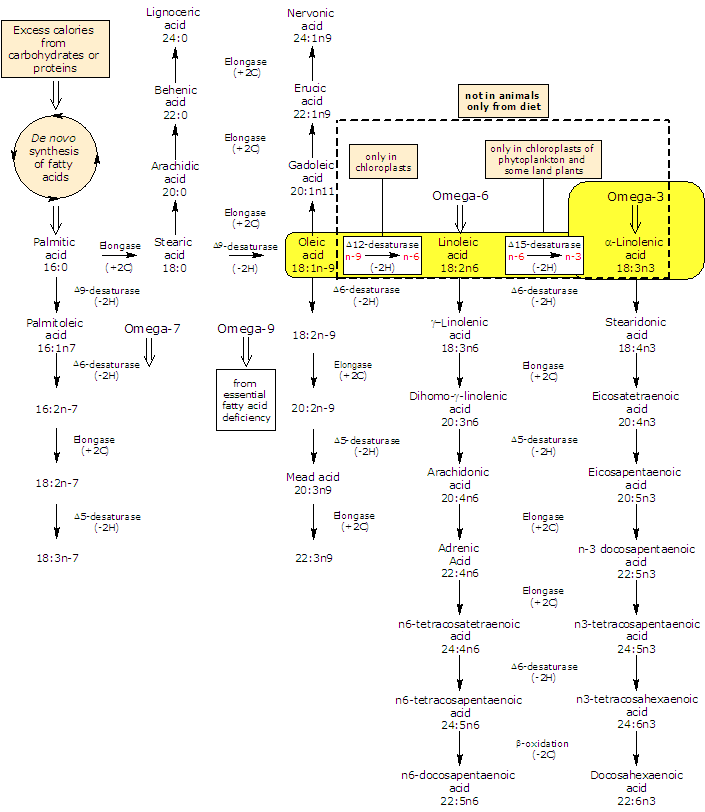
ALA and linoleic acid are the two main unsaturated fatty acids produced by plants. As both the desaturases lack in animals, they are essential fatty acids, that is, they must be obtained from plant foodstuff or animals that eat them.
ALA and omega-3
In animals ALA is the precursor for the production of omega-3 polyunsaturated fatty acids: it can be elongated and desaturated, in a cascade of reaction, to form very long fatty acids such as eicosapentaenoic acid or EPA and docosapentaenoic acid or DPA.
But, the efficiency of synthesis decreases down the cascade: conversion of alpha-linolenic acid to EPA is limited (the activity of Δ6-desaturase is the rate limiting in the conversion of α-linolenic acid to EPA in humans) and to docosahexaenoic acid (DHA) is even more restricted than that of EPA.
Food sources of α-linolenic acid
Modern agriculture has greatly enriched linoleic acid plant content but not of α-linolenic acid, so over the last 100 years its average dietary content has declined significantly.
alpha-Linolenic acid occurs as glycerol ester in many seed oils (as a minor component) like those of flaxseed, perilla, hemp and, to a lesser extent, of rapeseed (canola), soybeans and walnuts; it can also be obtained from green leaves (from photosynthetic tissues: chloroplast) of broadleaf plants and from animals that eat greens. The two major sources for human food are soybean and canola.
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008