Caprylic acid is a saturated fatty acid with a linear aliphatic chain consisting of eight carbon atoms.[10]
The main sources in the human diet are dairy products, as well as coconut oil and palm kernel oil, where it is found not in free form but as a component of triglycerides.[15]
In the digestive tract, by the action of lipases, caprylic acid is released from triglycerides, absorbed by passive diffusion from enterocytes and transported via the portal circulation to the liver, where it is mostly metabolized for energy.[13][14]
In mammals, it can be synthesized de novo in the mammary gland after childbirth, or produced by beta-oxidation cycles of fatty acids with longer chains, in the liver.[3][4]
The applications of caprylic acid range from pharmaceuticals to its use in the nutrition of individuals affected by conditions such as acute pancreatitis, or in the feeding of premature infants.[1][8][9]
Contents
- Chemical properties
- Food sources
- Intestinal absorption
- Transport
- Biosynthesis
- Caprylic acid and ghrelin
- Applications
- References
Chemical properties
Caprylic acid has a molecular weight of 144.21 g/mol, a molecular formula C8H16O2, a condensed formula CH3(CH2)6COOH, and the abbreviation 8:0. According to the IUPAC nomenclature, its systematic name is octanoic acid.[10]
At physiological pH, the free fatty acid predominantly exists in ionized form due to its pKa 4.89. Its conjugated base is known as octanoate or caprylate ion, with condensed formula CH3(CH2)6COO–.[9]
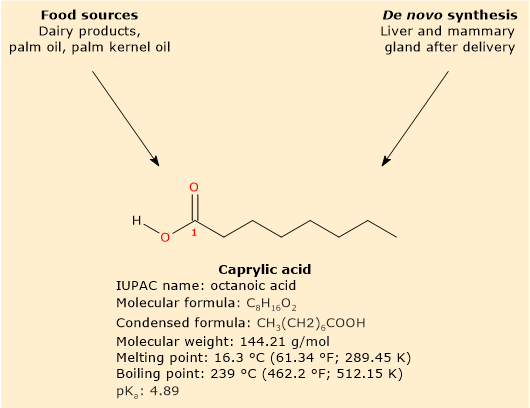 The absence of double bonds in the carbon chain places caprylic acid in the group of saturated fatty acids. Furthermore, since the number of carbon atoms in the chain is equal to 8, it can also be included in the group of medium-chain fatty acids or MCFAs, namely, fatty acids that have from 6 to 12 carbon atoms in the hydrocarbon chain.[1]
The absence of double bonds in the carbon chain places caprylic acid in the group of saturated fatty acids. Furthermore, since the number of carbon atoms in the chain is equal to 8, it can also be included in the group of medium-chain fatty acids or MCFAs, namely, fatty acids that have from 6 to 12 carbon atoms in the hydrocarbon chain.[1]
Caprylic acid is an isomer of valproic acid, an antiepileptic drug used in the treatment of epilepsy, bipolar disorder, and the prevention of migraine.
It is minimally soluble in water (789 mg/L at 30 °C) but soluble in ethanol. At room temperature, it appears as a colorless to light yellow liquid with an unpleasant odor reminiscent of goats.
Its melting point is 16.3 °C (325.4 °F; 436.15 K), while its boiling point is 239 °C (461.2 °F; 512.15 K).[10]
In triglycerides, it is esterified mainly in the sn-1 and sn-3 positions.[7]
Food sources
In the human diet, milk and dairy products, such as butter and cheese, as well as coconut and palm kernel oils, are the primary sources of caprylic acid.[15]
In bovine and human milk, together with other MCFAs, it constitutes approximately 7-17 percent and 9-28 percent of total fatty acids.[14] Specifically, in bovine milk, caproic, caprylic and capric acids constitute 4-12 percent of the fatty acids, with caprylic acid at 1.5-3.5 percent, while lauric acid 2.5-4 percent.[15][16]
During the maturation of hard cheeses, small amounts of triglycerides are hydrolyzed releasing fatty acids such as butyric, caproic, caprylic, and capric acids, which contribute to the cheese’s distinctive aroma.[3]
In coconut oil, it constitutes approximately 5-10 percent of the total fatty acids present, while in palm kernel oil 3-5 percent.[6]
Intestinal absorption
In the digestive tract, triglycerides are hydrolyzed by the action of specific lipases releasing fatty acids, which are then absorbed by the enterocytes.
The passage of caprylic acid through the plasma membrane of the enterocyte, as well as other cell membranes it encounters, including the inner mitochondrial membrane, is independent of specific protein transporters and occurs via passive diffusion. This means that even its entry into the mitochondrial matrix, where it is used for energy, is independent of the carnitine shuttle, similar to the other MCFAs and short-chain fatty acids (SCFAs).[13]
These transport mechanisms are likely responsible for the faster metabolic utilization rate of caprylic acid, other MCFAs, and SCFAs compared to long-chain fatty acids, which require the carnitine shuttle.[12][13]
Transport
The fraction of caprylic acid not used by the enterocyte leaves the cell without being incorporated into chylomicrons. Instead, it passes directly into the capillaries of the portal circulation where it is mostly bound to the plasma protein albumin. From there, it reaches the liver, where it is absorbed and rapidly metabolized.[14]
Because it is not incorporated into chylomicrons, it bypasses the lymphatic circulation and subsequent entry into the systemic circulation, making it unlikely for the fatty acid to be stored in adipose tissue.[1][8]
Biosynthesis
Mammals possess the enzymatic set for the synthesis of both SCFAs and MCFAs, and therefore also of caprylic acid. The organs most involved are the liver and mammary gland.
Hepatic synthesis primarily involves peroxisomal beta-oxidation cycles of fatty acids with a chain longer than the fatty acid being synthesized. During each cycle, the carbon chain is shortened by two carbon atoms compared to the incoming fatty acid.[11] A thioesterase hydrolyzes the thioester bond of the acyl-CoA, leading to the release of the fatty acid, which can then reach the cytosol.[4]
The mammary gland is able to synthesize de novo caprylic acid and the other MCFAs. Synthesis is triggered by childbirth, as demonstrated by the analysis of glandular secretion, which shows the presence of lipids in much lower quantities than in colostrum and milk.[3] Furthermore, both in humans and in other species, the rate of synthesis of other MCFAs increases with the duration of lactation.[3] In the mammary gland, de novo synthesis of medium-chain fatty acids seems to occur due to the presence of thioesterase II (EC 3.1.2.20), an acyl-CoA hydrolase capable of stopping the elongation of the aliphatic chain before reaching 16 carbon atoms, namely palmitic acid, as thioesterase I (EC 3.1.2.2) does in other tissues.[5]
Caprylic acid and ghrelin
Caprylic acid can act as an acyl group donor in the acylation reaction of ghrelin, an orexigenic hormone, that is, appetite-stimulating hormone.
Recent studies suggest that reducing the level of circulating octanoylated ghrelin, either by inhibiting the activity of the ghrelin O-acyltransferase enzyme (EC:2.3.1.-) or by limiting dietary caprylic acid, could be a potential strategy in combating obesity.[7][8]
Applications
Caprylic acid has a wide range of applications.
In the pharmaceutical industry, it is used as an albumin stabilizer during pasteurization, as a selective precipitant for the isolation of IgG from serum or plasma, and as an antimicrobial agent in the treatment of infections. All these effects have been linked to its ability to bind proteins and lipids.[9]
Due to its transport mechanisms, caprylic acid and other MCFAs, administered as medium-chain triglyceride oils (MCTs), are particularly important for the nutrition of individuals with pancreatic insufficiency, fat malabsorption, impaired chylomicron transport, and premature infants.[1][8]
In medical research, caprylic acid appears to have a potential as an adjuvant in the treatment of high-grade glial tumors. These tumor cells, due to defective mitochondria, rely on anaerobic glycolysis even in the presence of oxygen and are unable to metabolize ketone bodies. A treatment based on therapeutic ketosis has therefore been proposed for glioblastoma multiforme. In this context, caprylic acid is among the best precursors of ketone bodies and, in vitro and at high doses, has been shown to induce tumor necrosis.[2]
References
- ^ a b c d Akoh C.C., Min D.B. Food lipids: chemistry, nutrition, and biotechnology. 3th Edition. 2008. doi:10.1201/9781420046649
- ^ Altinoz M.A., Ozpinar A., Seyfried T.N. Caprylic (octanoic) acid as a potential fatty acid chemotherapeutic for Glioblastoma. Prostaglandins Leukot Essent Fatty Acids 2020;159:102142. doi:10.1016/j.plefa.2020.102142
- ^ a b c d Chow C.K. Fatty acids in foods and their health implication. 3th Edition. 2008. doi:10.1201/9781420006902
- ^ a b Hunt M.C., Siponen M.I., Alexson S.E. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim Biophys Acta 2012;1822(9):1397-410. doi:10.1016/j.bbadis.2012.03.009
- ^ Hunt M.C., Yamada J., Maltais L.J., Wright M.W., Podesta E.J., Alexson S.E. A revised nomenclature for mammalian acyl-CoA thioesterases/hydrolases. J Lipid Res 2005;46(9):2029-32. doi:10.1194/jlr.E500003-JLR200
- ^ Ibrahim N.A., Kuntom A., Tang T.S., Siew W.L. Current status of malaysian crude palm kernel oil characteristics. Oil Palm Bull 2003;47:15–27.
- ^ a b Lemarié F., Beauchamp E., Drouin G., Legrand P., Rioux V. Dietary caprylic acid and ghrelin O-acyltransferase activity to modulate octanoylated ghrelin functions: What is new in this nutritional field? Prostaglandins Leukot Essent Fatty Acids 2018;135:121-127. doi:10.1016/j.plefa.2018.07.009
- ^ a b c d Lemarié F., Beauchamp E., Legrand P., Rioux V. Revisiting the metabolism and physiological functions of caprylic acid (C8:0) with special focus on ghrelin octanoylation. Biochimie 2016;120:40-8. doi:10.1016/j.biochi.2015.08.002
- ^ a b c Li Y. The application of caprylic acid in downstream processing of monoclonal antibodies. Protein Expr Purif 2019;153:92-96. doi:10.1016/j.pep.2018.09.003
- ^ a b c National Center for Biotechnology Information. PubChem Compound Summary for CID 379, Octanoic Acid. https://pubchem.ncbi.nlm.nih.gov/compound/Octanoic-Acid. Accessed Jan. 2, 2025.
- ^ Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012.
- ^ Pégorier J.P., Duée P.H., Herbin C., Laulan P.Y., Bladé C., Peret J., Girard J. Fatty acid metabolism in hepatocytes isolated from rats adapted to high-fat diets containing long- or medium-chain triacylglycerols. Biochem J 1988;249(3):801-6. doi:10.1042/bj2490801
- ^ a b c Roopashree P.G., Shetty S.S., Kumari N.S. Effect of medium chain fatty acid in human health and disease. J Funct Foods 2021;87:104724. doi:10.1016/j.jff.2021.104724
- ^ a b c Schönfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57(6):943-54. doi:10.1194/jlr.R067629
- ^ a b c Jadhav H.B., Annapure U.S. Triglycerides of medium-chain fatty acids: a concise review. J Food Sci Technol 2023;60(8):2143-2152. doi:10.1007/s13197-022-05499-w
- ^ Jensen R.G. The composition of bovine milk lipids. J Dairy Sci 2002;85:295-350. doi:10.3168/jds.S0022-0302(02)74079-4