Elaidic acid (18 carbon atoms) was first obtained by Poutet J.J.E. in 1819; in 1832 Boudet F. studying accurately Poutet’s work isolated a fatty acid that he named “acide élaidique”, from the Greek word elais, meaning olive.
Until 1952 elaidic acid was only known as laboratory product; Swern D. et al. demonstrated its presence in beef fat.
Later it was shown that in ruminants it results from the bacterial hydrogenation of unsaturated fatty acids in the first stomach of the animals.
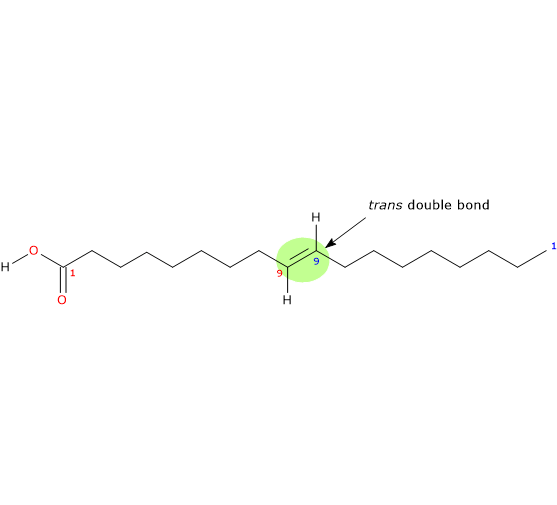
It is a monounsaturated (one trans double bond; shorthand nomenclature cannot be used to name trans fatty acids) fatty acid member of the sub-group called long chain fatty acids (LCFA) (from 14 to 18 carbon atoms) and is the trans-isomer of oleic acid.
PROPERTIES
Molecular weight: 282.46136 g/mol
Molecular formula: C18H34O2
IUPAC name: (E)-octadec-9-enoic acid
CAS registry number: 112-79-8
PubChem: 637517
In purified form it is a white crystalline fatty acid insoluble in water, with melting point at 44.5-45.5 °C (112.1-113.9 °F; 317.65-318.65 K) and boiling point at 288 °C (550.4 °F; 561.15 K) at 100 mm Hg.
OTHER NAMES
trans-oleic acid
9-octadecenoic acid
(E)-oleic acid
trans-9-octadecenoic acid
trans-elaidic acid
9-octadecenoic acid, (E)-
(9E)-octadecenoic acid
9-trans-octadecenoic acid
D9-trans-octadecenoic acid
trans-D9-octadecenoic acid
(9E)-octadec-9-enoic acid
trans-delta(9)-octadecenoic acid
Food sources of elaidic acid
Elaidic acid is the major trans fatty acid in margarine, fried foods and partially hydrogenated oils and also occurs in small amount (about 0.1 %of total fatty acids) in caprine and cow milk, always as glycerol ester.
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008