Stearidonic acid or SDA (18 carbon atoms) is an essential polyunsaturated fatty acid or PUFA, with four cis (Z) double bounds (the first one from the methyl end is in omega-3 (ω-3) or n-3 so in shorthand 18:4n-3), member of the sub-group called long chain fatty acids (LCFAs), from 14 to 18 carbon atoms.
PROPERTIES
Molecular weight: 276.41372 g/mol
Molecular formula: C18H28O2
IUPAC name: (6Z,9Z,12Z,15Z)-octadeca-6,9,12,15-tetraenoic acid
CAS registry number: 20290-75-9
PubChem: 5312508
OTHER NAMES
SDA
moroctic acid
cis-6,9,12,15-octadecatetraenoic acid
6,9,12,15-octadecatetraenoic acid
(6Z,9Z,12Z,15Z)-octadecatetraenoic acid
all-cis-6,9,12,15-octadecatetraenoic acid
6,9,12,15-octadecatetraenoate
6,9,12,15-cis-octadecatetraenoic acid
18:4n-3
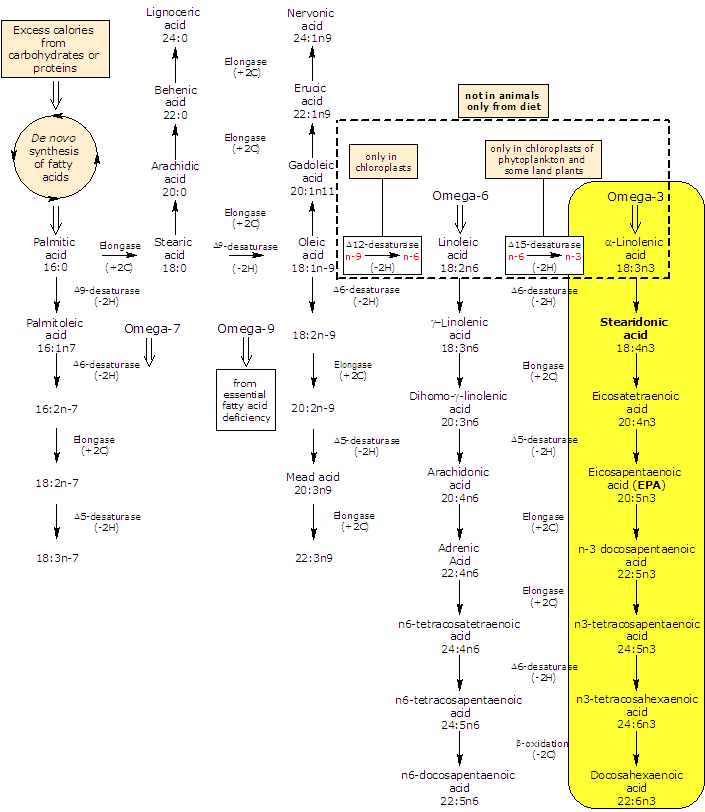
Synthesis and metabolism of stearidonic acid
Stearidonic acid is produced de novo from alpha-linolenic acid in a desaturation reaction catalyzed by the enzyme Δ6-desaturase, a reaction extremely inefficient in humans (as shown previously in rodents).
On the other hand, dietary and from de novo synthesis stearidonic acid is efficiently converted in eicosapentaenoic acid or EPA and docosapentaenoic acid or DPA by the subsequent actions of elongase (it catalyzes the addition of two carbon atoms from glucose metabolism to lengthen the fatty acid chain) and Δ5-desaturase and for this reasons its use may be a valuable tool for increasing EPA and docosahexaenoic acid (DHA) tissue concentrations.
The further metabolism to DHA depends again on Δ6-desaturase (followed by a β-oxidation) and for this reasons this conversion is limited.
Food sources
It occurs as glycerol ester in the leaf lipids of borage (Borago Offcianalis L., of the Boraginaceae) and in blackcurrant seed oil (3.5%) (Ribes nigrum, of the Saxifragaceae).
References
- Akoh C.C. and Min D.B. “Food lipids: chemistry, nutrition, and biotechnology” 3th ed. 2008
- Chow Ching K. “Fatty acids in foods and their health implication” 3th ed. 2008
- James J.M., Ursin M.V., and Cleland G.L. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr 2003;77:1140-1145. doi:10.1093/ajcn/77.5.1140