Arachidonic acid is a polyunsaturated fatty acid with a chain of 20 carbon atoms and 4 cis double bonds at positions 5, 8, 11, and 14.[13]
Its major dietary sources are eggs, meat, and fish.[2]
Animals can synthesize arachidonic acid from linoleic acid, the precursor to omega-6 polyunsaturated fatty acids.[1]
Arachidonic acid is found in cell membranes, where it is esterified within phospholipids acting as a structural component. The highest content, expressed as a percentage of phospholipid fatty acids, is found in platelets.[2][16]
It is the precursor of many bioactive lipid mediators, which are responsible for various functional effects attributed to the fatty acid. Examples are eicosanoids, namely, leukotrienes, prostaglandins, prostacyclins, and thromboxanes, some with anti-inflammatory effects and others with pro-inflammatory effects.[9]
In healthy adult individuals, an intake of up to 1.5 g/day does not appear to have adverse effects and may help reduce the risk of cardiovascular disease.[14]
Contents
- Chemical properties
- Food sources
- Biosynthesis
- Cellular distribution
- The arachidonic acid metabolome
- Biosynthesis of Osbond acid
- Eicosanoids
- Arachidonic acid: harmful or helpful?
- References
Chemical properties
Arachidonic acid has a molecular weight of 304.47 g/mol, a molecular formula C20H32O2, and the abbreviation 20:4n-6. According to the IUPAC nomenclature, its systematic name is (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid.[13]
At physiological pH, the free fatty acid predominantly exists in ionized form due to its pKa 4.82. Its conjugated base is known as arachidonate.[10]
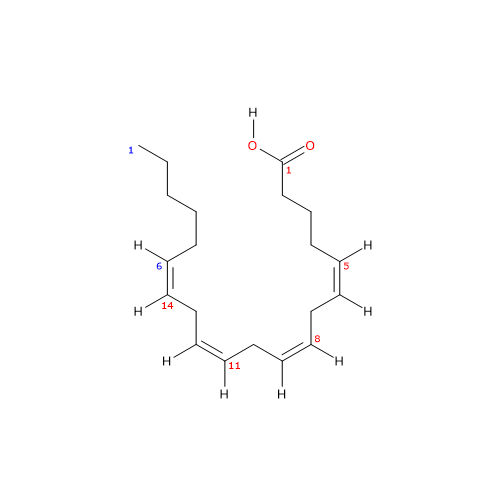
The presence of four cis double bonds places arachidonic acid in the group of unsaturated fatty acids. Furthermore, because the first double bond is at carbon 6 from the methyl end, the molecule belongs to the group of omega-6 polyunsaturated fatty acids.[1]
Since the chain contains 20 carbon atoms, it is classified as a long-chain fatty acid, defined as having carbon chains of 13 to 21 atoms.
It is practically insoluble in water (0.00015 g/L).[10]
At room temperature, it appears as an oily liquid with a density of 0.922 g/ml.[20]
Its melting point is -49.5 °C (-57.1 °F; 223.6 K), while its boiling point at 0.15 mm Hg is 170 °C (338 °F; 443.1 K).[13]
In cells, it is present both in phospholipids of cell membranes, generally esterified in the sn-2 position, and in triglycerides of fat.[16]
Food sources
Important sources of arachidonic acid are meat, eggs, and fish.[2]
In meat, it is present both in lean cuts, where it is concentrated in membrane phospholipids, and in visible fat. Pork fat is particularly rich, containing about 1.8 mg/g, while among lean cuts, duck is the richest with about 1 mg/g. Meats, both lean and fatty cuts, with the lowest quantities are those of beef and lamb. Notable fish sources include tuna and salmon, each containing about 3 mg/g, and herring, with 0.4 mg/g.[12]
The average daily intake in an adult following a Western diet has been estimated to be between 50 and 300 mg.[2]
Biosynthesis
Arachidonic acid can be synthesized from endogenous precursors, particularly linoleic acid, which is one of the two essential fatty acids for animals, along with alpha-linolenic acid.[4]
Its synthesis occurs in the endoplasmic reticulum and involves two desaturation steps and one elongation step.[1][4]
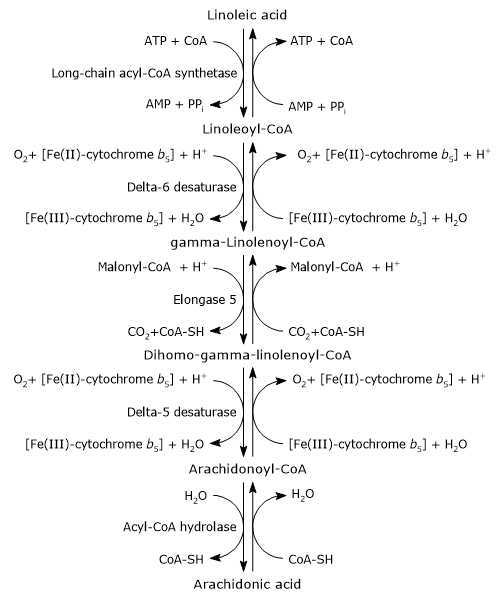
In the first step, linoleic acid is activated by being joined to coenzyme A (CoA-SH). The reaction is catalyzed by a long-chain acyl-CoA synthetase (EC 6.2.1.3), at the expense of one molecule of ATP.[1]
Linoleoyl-CoA is desaturated to gamma-linolenoyl-CoA. The reaction is catalyzed by delta-6 desaturase (EC 1.14.19.3). The enzyme introduces a cis double bond at carbon 6 from the carboxyl end of acyl-CoAs.[11] Delta-6 desaturase is the rate-limiting enzyme of the pathway and is affected by nutritional deficiencies and by inflammatory processes.[4][8]
Gamma-linolenoyl-CoA is elongated by two carbon atoms to form dihomo-gamma-linolenoyl-CoA. The reaction is catalyzed by elongase 5 or elongation of very long chain fatty acids protein 5 (EC 2.3.1.199), and malonyl-CoA is the donor of the acetyl group.[4]
Dihomo-gamma-linolenoyl-CoA is desaturated to arachidonoyl-CoA. The reaction is catalyzed by delta-5 desaturase or acyl-CoA (8-3)-desaturase (EC 1.14.19.44) that introduces a cis double bond at carbon 5 of acyl-CoAs containing a double bond at position 8 from the carboxyl end.[11]
Delta-5 desaturase was suggested to be potentially rate-limiting during supplementation with gamma-linolenic acid. Consequently, most of the dihomo-gamma-linolenic acid formed in the previous reaction is inserted at the sn-2 position of phospholipids, similar to arachidonic acid. Its activity is influenced by nutritional and environmental factors.[16]
In the last step, the thioester bond of arachidonoyl-CoA is hydrolyzed with the release of arachidonic acid and coenzyme A. The reaction is catalyzed by an acyl-CoA hydrolase (EC 3.1.2.20).[4]
Competition for elonagses and desaturases
The elongases and desaturases that catalyze the conversion of linoleic acid to arachidonic acid are also involved in the synthesis of omega-3, omega-7, and omega-9 polyunsaturated fatty acids. Omega-3 polyunsaturated fatty acids seem to be the preferred substrates for desaturases; however, the synthesis of omega-6 polyunsaturated fatty acids predominates due to the high dietary intake of linoleic acid.
Role of linoleic acid
Linoleic acid is the major polyunsaturated fatty acid in the Western diet.[16]
Based on animal studies, it has been assumed that in humans, increasing the intake of linoleic acid could enhance the synthesis of arachidonic acid. For this reason, it has been recommended to limit dietary linoleic acid intake to reduce tissue levels of arachidonic acid.[23]
However, it has been shown that there is no dose-response effect between the intake of dietary linoleic acid and arachidonic acid tissue levels in subjects consuming a Western-type diet. These data are also supported by the observation that, in adults, the rate of conversion of plasma/serum linoleic acid to arachidonic acid is very low, being between 0.3 percent and 0.6 percent.[5] The underlying reason is unclear. It seems that the limiting factor is not the saturation of tissue arachidonic acid content but rather the reaction catalyzed by delta-6 desaturase. For example, levels of arachidonic acid in blood phospholipids increase following the administration of gamma-linolenic acid and arachidonic acid itself.[16]
Cellular distribution
Mammalian cells and tissues are rich in arachidonic acid, which is predominantly found in membrane phospholipids, mainly esterified in the sn-2 position.[16]
Arachidonic acid is one of the most important unsaturated fatty acids in cell membranes, where it acts as a structural component, similar to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). It helps regulate membrane fluidity, selective permeability, and mechanical flexibility.[24]
In adult humans following a Western diet, arachidonic acid levels vary across cell types. The highest concentration is found in platelets, constituting about 25 percent of phospholipid fatty acids. Lower levels are observed in mononuclear cells (22 percent), liver (20 percent), erythrocytes (17 percent), skeletal muscle (17 percent), neutrophils (15 percent), and cardiac muscle (9 percent).[2]
The arachidonic acid metabolome
Arachidonic acid can be converted into numerous bioactive derivatives.[9]
The analysis of the metabolome of arachidonic acid highlights the presence of a constellation of metabolites, such as polyunsaturated fatty acids with a higher degree of unsaturation and longer carbon chains, as well as many bioactive lipids, some with pro-inflammatory activities, and others with anti-inflammatory activities or able to promote the resolution of inflammatory injuries.[9][24]
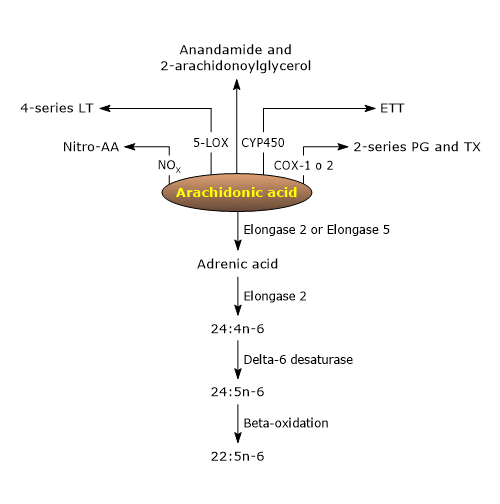
Among these bioactive lipid mediators, there are ligands for endocannabinoid receptors, such as anandamide and 2-arachidonoylglycerol, nitrated arachidonic acid, an inflammatory and vascular signaling molecule, with eicosanoids being particularly notable.[9]
Biosynthesis of Osbond acid
Arachidonic acid can be converted to Osbond acid (22:5n-6) through four reactions, the first three occurring in the endoplasmic reticulum and the last in peroxisomes.[1][4]
- In the first step, arachidonic acid is elongated to form adrenic acid (22:4n-6) in the reaction catalyzed by either elongase 2 (Elovl2, EC 2.3.1.199) or elongase 5 (Elovl5, EC 2.3.1.199).
- Adrenic acid is elongated to tetracosatetraenoic acid (24:4n-6) in the reaction catalyzed by elongase 2.
- Tetracosatetraenoic acid is desaturated to tetracosapentaenoic acid (24:5n-6) in the reaction catalyzed by delta-6 desaturase.
- In the last step, tetracosapentaenoic acid undergoes peroxisomal beta-oxidation to form Osbond acid or n-6 docosapentaenoic acid (n-6 DPA).
Eicosanoids
Eicosanoids, namely prostaglandins, leukotrienes, prostacyclins, and thromboxanes, are a group of oxygenated 20-carbon fatty acids that act as mediators with autocrine and paracrine activity. Their main precursor is arachidonic acid; others are dihomo-gamma-linolenic acid, EPA, and DHA.[1][24]
Their biosynthesis, like that of the other bioactive lipid compounds seen previously, is preceded by the release of arachidonic acid from membrane phospholipids in a reaction catalyzed by phospholipase A2 (EC 3.1.1.4).[6] A second minor pathway for the release of the fatty acid is the hydrolysis of diacylglycerol (DAG), in a reaction catalyzed by DAG lipase (EC 3.1.1.-).[21]
The pathways leading to the biosynthesis of eicosanoids from arachidonic acid are collectively known as the “arachidonate cascade”, comprising three major pathways named after the enzyme catalyzing the first step.[4]
The cyclooxygenase pathway
In the cyclooxygenase pathway, the first reaction is catalyzed by prostaglandin-endoperoxide synthase (PTGS) or cyclooxygenase (EC 1.14.99.1).
There are two cyclooxygenase isoenzymes, COX-1 and COX-2, also known as PTGS1 and PTGS2, or prostaglandin G/H synthase 1 and 2.[18]
COX-1 is the constitutive enzyme and is present in most cells, except red blood cells.
COX-2 is the inducible form. It is constitutively expressed in uninflamed tissues and organs, such as heart, kidney, the vasculature, gastric epithelium, and brain, but may be induced by inflammatory stimuli in epithelial cells, white blood cells, and smooth muscle cells.[7] This pathway leads to the synthesis of 2-series prostaglandins (PT), and their derivatives 2-series thromboxanes (TX), so-called because they have two double carbon-carbon bonds in their side chains.
Some of these molecules have pro-inflammatory activity, such as prostaglandin E2 or PGE2, others anti-inflammatory activity, such as thromboxane A2 or TXA2.[17]
The lipoxygenase pathway
In the lipoxygenase pathway, the first reaction is catalyzed by the arachidonate 5-lipoxygenase or 5-lipoxygenase (5-LOX, EC 1.13.11.34). This reaction leads to the formation of 5(S)-hydroperoxy eicosatetraenoic acid or 5(S)-HPETE.[9]
This pathway leads to the synthesis of 4-series leukotrienes (LT), so-called because they have four double carbon-carbon bonds in their side chains. LT are pro-inflammatory signaling molecules, such as leukotriene B4 or LTB4, and are the precursors of the lipoxins (LX), eicosanoids with anti-inflammatory properties, such as lipoxin A4 or LXA4 and lipoxin B4 or LXB4.[15]
The epoxygenase pathway
In the epoxygenase pathway, the first reaction is catalyzed by a cytochrome P450 epoxygenase.[9]
This pathway leads to the synthesis of epoxyeicosatrienoic acids (EETs), molecules with vasorelaxant activity, profibrinolytic as well as anti-inflammatory effects. In vivo, EETs are rapidly metabolized to dihydroxyeicosatrienoic acids or DHETs, in reactions catalyzed by a soluble epoxy hydrolase. DHETs are less active than EETs.[22]
Two other series of reactions are grouped into the epoxygenase pathway. They are initiated by cytochrome P450 mixed-function oxidases. These enzymes lead to the synthesis of hydroperoxyeicosatetraenoic acids or HPETEs, some of which are epimers of lipoxygenase-catalyzed products, and omega-oxidized monohydroxyeicosatetraenoic acidsi.[21]
Arachidonic acid: harmful or helpful?
The idea that arachidonic acid may be harmful is derived from the following observations.
- Numerous arachidonic acid-derived mediators are implicated in various pathologies due to their pro-inflammatory action.
- Free arachidonic acid itself acts as an immunosuppressant, induces inflammatory responses, and serves as a potent platelet aggregator.
- The health benefits of omega-3 polyunsaturated fatty acids often arise from their antagonistic effects on arachidonic acid: they partially replace it in membrane phospholipids and inhibit its metabolism into eicosanoids.
- A study published in 1975, in which 6 g/day of arachidonic acid were administered to healthy volunteers, was halted due to a marked increase in platelet aggregation ex vivo, a pro-thrombotic event.[19]
However, it is important to keep in mind that the aforementioned effects were only observed in certain tissues or cells, and often under non-physiological conditions, such as the study conducted in 1975. Therefore these effects, as well as their relevance under physiological conditions, remain to be clearly defined.[19]
Conversely, in subjects receiving 1.5 g/day of arachidonic acid, no effects were observed on inflammatory markers, immune functions, platelet reactivity, and bleeding time. These findings indicate that arachidonic acid intake of up to 1.5 g/day in healthy adults does not appear to have adverse effects.[14]
Finally, it is important to emphasize that infant formulas containing arachidonic acid in combination with DHA are associated with improved growth and development. In premature infants, these formulas support immune system development similar to that of breastfed infants and also reduce the risk of necrotizing enterocolitis. Thus, the fatty acid appears to play a crucial role in the healthy growth and development of infants.[3]
References
- ^ a b c d e f Akoh C.C., Min D.B. Food lipids: chemistry, nutrition, and biotechnology. 3th Edition. 2008. doi:10.1201/9781420046649
- ^ a b c d e Calder P. C. Dietary arachidonic acid: harmful, harmless or helpful? Br J Nutr 2007; 98:451-453. doi:10.1017/S0007114507761779
- ^ Carlson S.E., Montalto M.B., Ponder D.L., Werkman S.H., Korones S.B. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr Res 1998;44(4):491-8. doi:10.1203/00006450-199810000-00005
- ^ a b c d e f g Chow C.K. Fatty acids in foods and their health implication. 3th Edition. 2008. doi:10.1201/9781420006902
- ^ Demmelmair H., Iser B., Rauh-Pfeiffer A., Koletzko B. Comparison of bolus versus fractionated oral applications of [13C]-linoleic acid in humans. Eur J Clin Invest 1999;29(7):603-9. doi:10.1046/j.1365-2362.1999.00477.x
- ^ Dennis E.A., Cao J., Hsu Y.H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 2011;111(10):6130-85. doi:10.1021/cr200085w
- ^ Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., Lipsky P.E. Cyclooxygenase in biology and disease. FASEB J 1998;12(12):1063-73.
- ^ Gregory M.K., Gibson R.A., Cook-Johnson R.J., Cleland L.G., James M.J. Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis. PLoS One 2011;6(12):e29662. doi:10.1371/journal.pone.0029662
- ^ a b c d e f Harris W.S., Shearer G.C. Omega-6 fatty acids and cardiovascular disease. Friend, not foe? Circulation 2014;130:1562-1564. doi:10.1161/CIRCULATIONAHA.114.012534
- ^ a b HMDB. Metabocard for arachidonic acid. https://hmdb.ca/metabolites/HMDB0001043
- ^ a b Lee J.M., Lee H., Kang S. 3 and Park W.J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 2016;8(1):23. doi:10.3390/nu8010023
- ^ Li D., Ng A, Mann N.J., Sinclair A.J. Contribution of meat fat to dietary arachidonic acid. Lipids 1998;33(4):437-440. doi:10.1007/s11745-998-0225-7
- ^ a b c National Center for Biotechnology Information. PubChem Compound Summary for CID 444899, Arachidonic Acid. https://pubchem.ncbi.nlm.nih.gov/compound/Arachidonic-Acid. Accessed Dec. 15, 2024.
- ^ a b Nelson G.J., Schmidt P.C., Bartolini G., Kelley D.S., Phinney S.D., Kyle D., Silbermann S., Schaefer E.J. The effect of dietary arachidonic acid on plasma lipoprotein distributions, apoproteins, blood lipid levels, and tissue fatty acid composition in humans. Lipids 1997;32(4):427-33. doi:10.1007/s11745-997-0056-6
- ^ Powell W.S., Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta 2015;1851(4):340-55. doi:10.1016/j.bbalip.2014.10.008
- ^ a b c d e f Rett B.S. and Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond) 2011;8:36. doi:10.1186/1743-7075-8-36
- ^ Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986-1000. doi:10.1161/ATVBAHA.110.207449
- ^ Rouzer C.A., Marnett L.J. Cyclooxygenases: structural and functional insights. J Lipid Res 2009;50 Suppl(Suppl):S29-34. doi:10.1194/jlr.R800042-JLR200
- ^ a b Seyberth H.W., Oelz O., Kennedy T., Sweetman B.J., Danon A., Frolich J.C., Heimberg M. and Oates J.A. Increased arachidonate in lipids after administration to man: effects on prostaglandin biosynthesis. Clin Pharmacol Ther 1975;18:521-529. doi:10.1002/cpt1975185part1521
- ^ Sigma Aldrich. Product information. Arachidonic acid. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/199/672/a9673pis.pdf
- ^ a b Sonnweber T., Pizzini A., Nairz M., Weiss G., Tancevski I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int J Mol Sci 2018;19(11):3285. doi:10.3390/ijms19113285
- ^ Spector A.A. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res 2009;50 Suppl(Suppl):S52-6. doi:10.1194/jlr.R800038-JLR200
- ^ Wall R., Ross R.P., Fitzgerald G.F., Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 2010;68(5):280-9. doi:10.1111/j.1753-4887.2010.00287.x
- ^ a b c Zhang Y., Liu Y., Sun J., Zhang W., Guo Z., Ma Q. Arachidonic acid metabolism in health and disease. MedComm (2020) 2023;4(5):e363. doi:10.1002/mco2.363