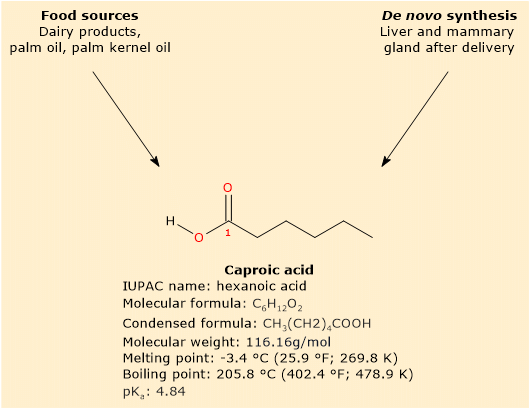
Caproic acid is a saturated fatty acid with a linear aliphatic chain of six carbon atoms.[8]
First isolated from butter by Chevreul M.E. in 1816, its name derives from the Latin word caper, meaning goat, due to its characteristic odor reminiscent of goats.[7]
Primarily found in dairy products, especially those derived from goat’s milk, it is not found in free form but rather as part of triglyceride.[3]
In the intestine, once released from triglycerides, caproic acid is rapidly absorbed thanks to its ability to cross cell membranes via passive diffusion. It is transported bound to albumin, through portal circulation, to the liver, where it is quickly metabolized for energy.[2] This characteristic makes it an important nutrient for individuals with specific energy requirements.[1]
In mammals, caproic acid is not only obtained through diet but can also be synthesized. This primarily occurs in the liver through beta-oxidation of longer fatty acids, or in the mammary gland through de novo synthesis after childbirth.[3][9]
Caproic acid has applications in various fields, including food, pharmaceutical, livestock, and energy sectors.[4][13][14]
Contents
- Chemical properties
- Dietary sources
- Intestinal absorption
- Transport
- Biosynthesis
- Applications
- References
Chemical properties
Caproic acid has a molecular weight of 116.16 g/mol, a molecular formula C6H12O2, a condensed formula CH3(CH2)4COOH, and the abbreviation 6:0.[8]
According to IUPAC nomenclature, its systematic name is hexanoic acid.
Caproic acid is slightly soluble in water but highly soluble in ethanol.[14]
At room temperature, it appears as a colorless or yellow oily liquid with a pungent odor reminiscent of goats.
Its melting point is -3.4 °C (25.9 °F; 269.8 K), and its boiling point is 205.8 °C (402.4 °F; 478.9 K).[8]
At physiological pH, the free fatty acid predominantly exists in this ionized form, given its pKa of 4.84.[12] Its conjugate base is known as hexanoate or caproate ion, with condensed formula CH3(CH2)4COO–.
 It provides approximately 7.5 kcal/g of energy.[1]
It provides approximately 7.5 kcal/g of energy.[1]
The absence of double bonds in the carbon chain places caproic acid in the group of saturated fatty acids, and, since the number of carbon atoms in the chain is equal to 6, it can also be included in the group of medium-chain fatty acids (MCFAs), fatty acids that have from 6 to 12 carbon atoms in the carbon chain.[1]
In triglycerides, caproic acid is primarily esterified in the sn-3 position.[1]
During cheese maturation, a small proportion of triglycerides are hydrolyzed, releasing fatty acids. Among these butyric, caproic, caprylic and capric acid contribute to the cheese’s distinctive aroma.[3]
Dietary sources
Caproic acid is present in various foods, particularly those rich in fats and oils, although typically in limited amounts, often less than one gram per 100 grams of product.
The primary sources are dairy products such as milk, butter, cheese, and cream. Among these, it is most abundant in goat’s milk and cheeses made from it.[3]
Small amounts of caproic acid are also found in coconut oil and palm kernel oil.[12]
In animal fats, it is only present in trace amounts.
Finally, caproic acid is one of the three fatty acids found in certain medium-chain triglyceride (MCT) oils.
Intestinal absorption
Like other fatty acids, caproic acid is present almost exclusively as a component of triglycerides and must be released to absorbed. Once hydrolyzed from the triglyceride, its slight solubility in water allows it to overcome the “water barrier” on the luminal surface of enterocytes. Its passage through the plasma membrane of the enterocyte, as with other cell membranes it encounters, is independent of specific protein transporters. This process occurs by passive diffusion and likely involves the protonated form of the molecule.[2] This means that even its entry into the mitochondrial matrix, where it is used for energy, is independent of the carnitine shuttle, similar to the other MCFAs and short-chain fatty acids (SCFAs).
These transport mechanisms are likely responsible for the faster metabolic rate of medium- and short-chain fatty acids compared to long-chain fatty acids, which require specific protein carriers.[10][11] For this reason, together with other MCFAs, it plays a particularly important role in the nutrition of premature babies and individuals with fat malabsorption.[1]
Transport
The fraction of caproic acid not used by the intestinal cell is not incorporated into chylomicrons but instead passes directly into the portal circulation. Here, it is transported mostly bound to albumin and delivered to the liver, where it is almost entirely absorbed and rapidly metabolized.[12]
Since caproic acid is not incorporated into chylomicrons, thus bypassing the lymphatic circulation and subsequent entry into the systemic circulation, and given the small amount present in food, it is unlikely to be stored in adipose tissue.
Biosynthesis
Although the main source of caproic acid is the diet, this fatty acid can also be synthesized in the tissues of mammals, including humans.
Synthesis occurs mainly in the liver through peroxisomal beta-oxidation cycles of fatty acids which shorten the carbon chain by two carbon atoms compared to the original fatty acid entering the cycle.[9] An acyl-CoA thioesterase then hydrolyzes the thioester bond, releasing the fatty acid which can subsequently reach the cytosol.[5]
Caproic acid, along with other medium-chain fatty acids, is also synthesized in the mammary gland but only after childbirth. Analyses of mammary gland secretions before childbirth have highlighted significantly lower quantities of lipids compared to colostrum and milk.[3] It therefore suggests that childbirth triggers its synthesis. In the mammary gland, de novo synthesis of fatty acids with aliphatic chains composed of 6 to 12 carbon atoms seems to occur due to the presence of thioesterase II (EC 3.1.2.20), an enzyme capable of halting the elongation of the carbon chain before it reaches 16 carbon atoms. This contrasts with other tissues, where thioesterase I (EC 3.1.2.2) stops the synthesis process once palmitic acid is produced.[6] In humans, as in other species, the synthesis rate of caproic acid and other MCFAs increases with the duration of breastfeeding.[3]
Applications
Caproic acid has a wide range of applications, from the food industry to the pharmaceutical, energy and livestock sectors.
- Food industry: it is used as an flavoring agent in spices and as a food additive, for example, in butter and bread.
- Pharmaceutical industry: it is used in the drug synthesis as a source of the ethyl group. For instance, it is employed in the production of zinc acetate caproate, a drug used in the treatment of gastric ulcers, and in the production of amino caproic acid, a haemostatic drug.[14]
- Animal husbandry: it is added to animal feed as a potential substitute for antibiotics. It promotes intestinal health and strengthens immunity.[4][13]
- Energy sector: it serves as a precursor for the production of biofuels, including diesel and aviation fuel, although it cannot be used directly as a fuel.[14]
References
- ^ a b c d e Akoh C.C., Min D.B. Food lipids: chemistry, nutrition, and biotechnology. 3th Edition. 2008. doi:10.1201/9781420046649
- ^ a b Charney A.N., Micic L., Egnor R.W. Nonionic diffusion of short-chain fatty acids across rat colon. Am J Physiol 1998;274(3):G518-24. doi:10.1152/ajpgi.1998.274.3.G518
- ^ a b c d e f Chow C.K. Fatty acids in foods and their health implication. 3th Edition. 2008. doi:10.1201/9781420006902
- ^ a b Desbois A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat Antiinfect Drug Discov 2012;7(2):111-22. doi:10.2174/157489112801619728
- ^ Hunt M.C., Siponen M.I., Alexson S.E. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim Biophys Acta 2012;1822(9):1397-410. doi:10.1016/j.bbadis.2012.03.009
- ^ Hunt M.C., Yamada J., Maltais L.J., Wright M.W., Podesta E.J., Alexson S.E. A revised nomenclature for mammalian acyl-CoA thioesterases/hydrolases. J Lipid Res 2005;46(9):2029-32. doi:10.1194/jlr.E500003-JLR200
- ^ Leray C. Contribution of Chevreul to lipid chemistry. OCL OILS FAT CROP LI 2023:30(9). doi:10.1051/ocl/2023006
- ^ a b c National Center for Biotechnology Information. PubChem Compound Summary for CID 8892, Caproic Acid. https://pubchem.ncbi.nlm.nih.gov/compound/Caproic-Acid. Accessed Dec. 22, 2024.
- ^ a b Nelson D.L., Cox M.M. Lehninger. Principles of biochemistry. 6th Edition. W.H. Freeman and Company, 2012.
- ^ Pégorier J.P., Duée P.H., Herbin C., Laulan P.Y., Bladé C., Peret J., Girard J. Fatty acid metabolism in hepatocytes isolated from rats adapted to high-fat diets containing long- or medium-chain triacylglycerols. Biochem J 1988;249(3):801-6. doi:10.1042/bj2490801
- ^ Roopashree P.G., Shetty S.S., Kumari N.S. Effect of medium chain fatty acid in human health and disease. J Funct Foods 2021;87:104724. doi:10.1016/j.jff.2021.104724.
- ^ a b c Schönfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57(6):943-54. doi:10.1194/jlr.R067629
- ^ a b Van Immerseel F., De Buck J., Boyen F., Bohez L., Pasmans F., Volf J., Sevcik M., Rychlik I., Haesebrouck F., Ducatelle R. Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl Environ Microbiol 2004;70(6):3582-7. doi:10.1128/AEM.70.6.3582-3587.2004
- ^ a b c d Yuan S., Jin Z., Ali A., Wang C., Liu J. Caproic acid-producing bacteria in chinese Baijiu brewing. Front Microbiol 2022;13:883142. doi:10.3389/fmicb.2022.883142